
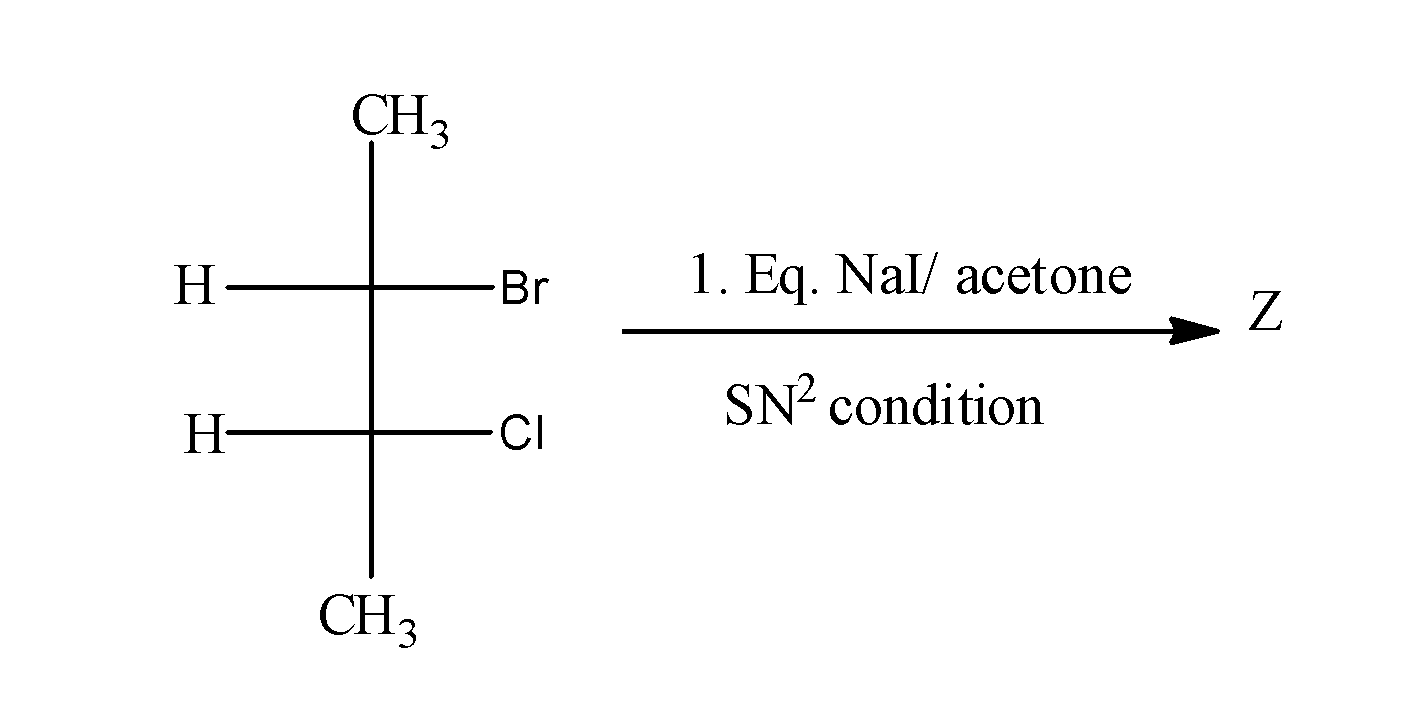
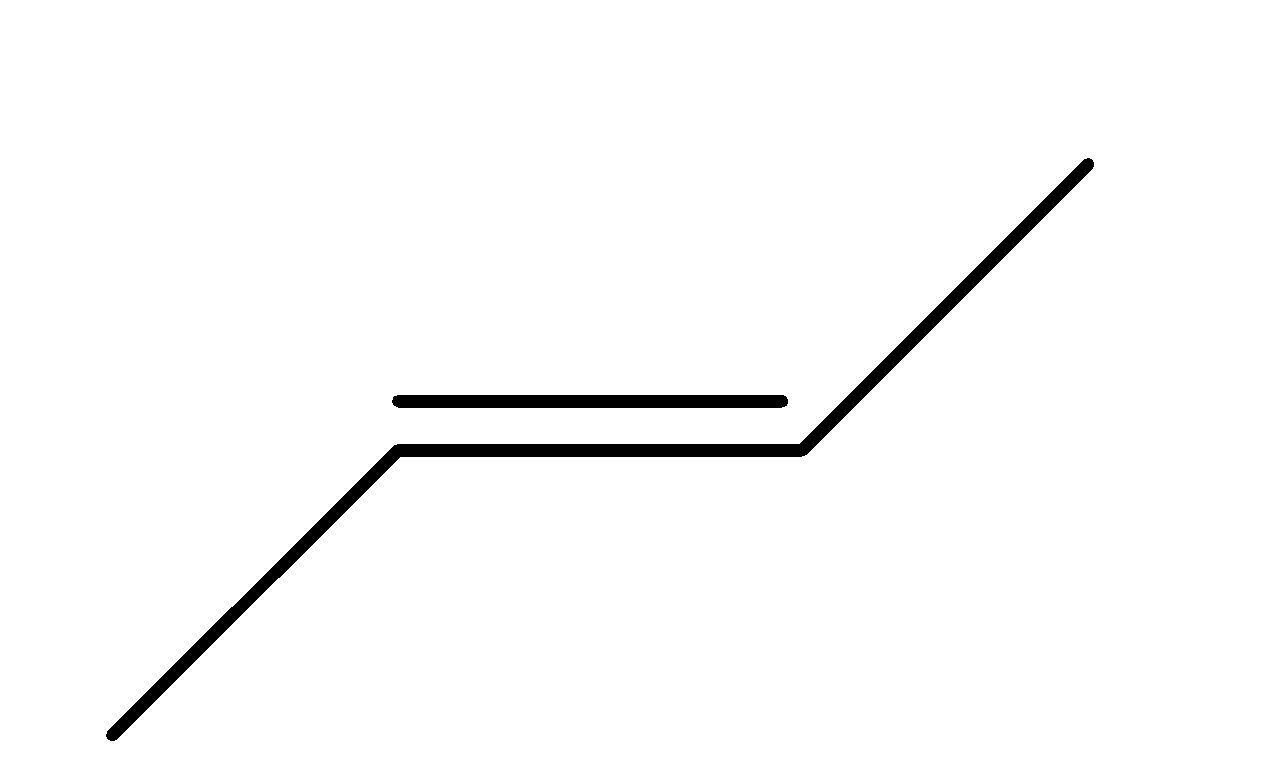
Z will be?
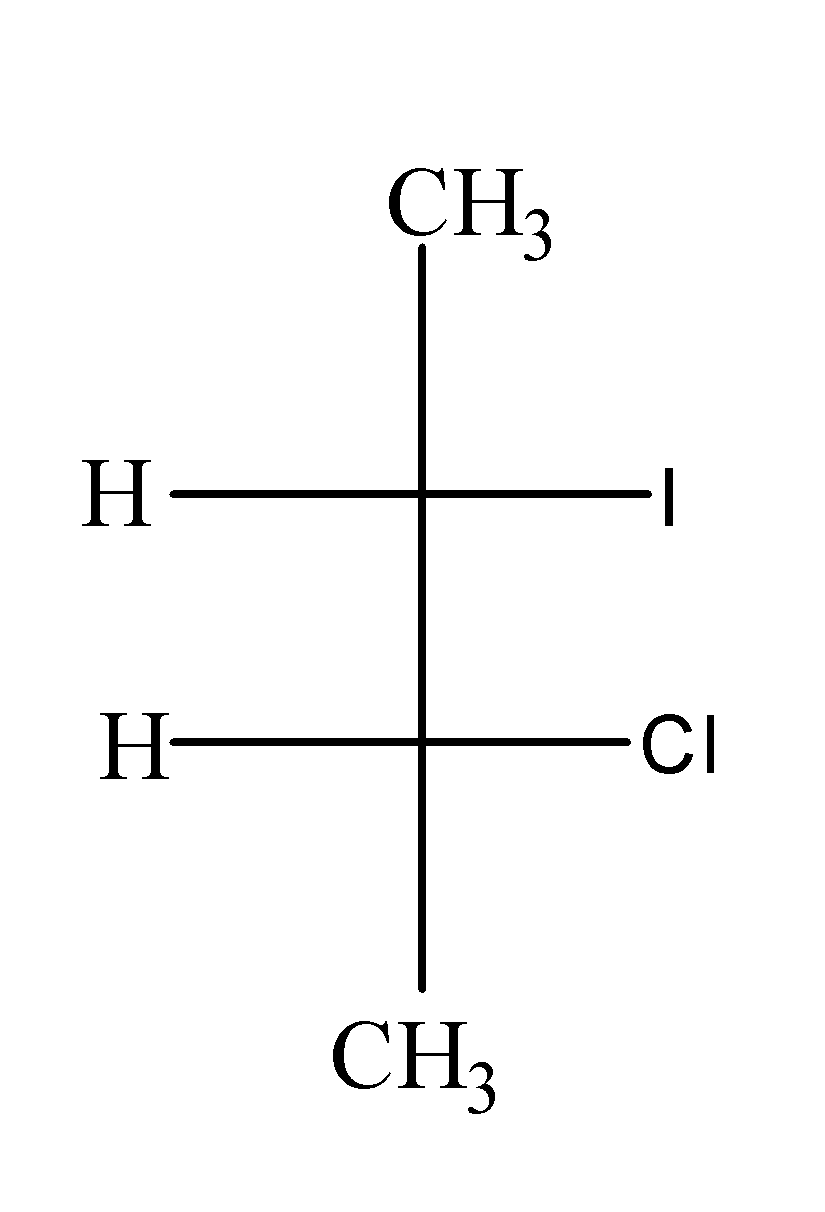
(a)
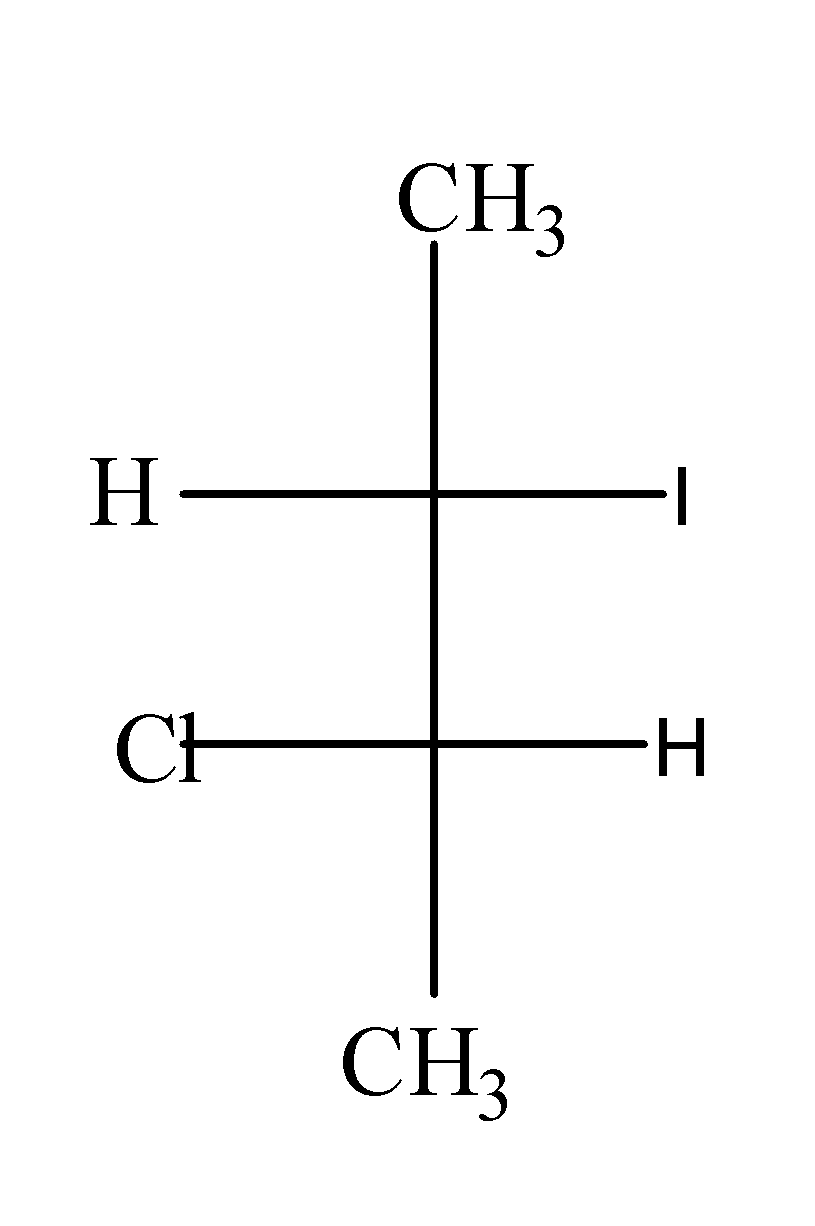
(b)
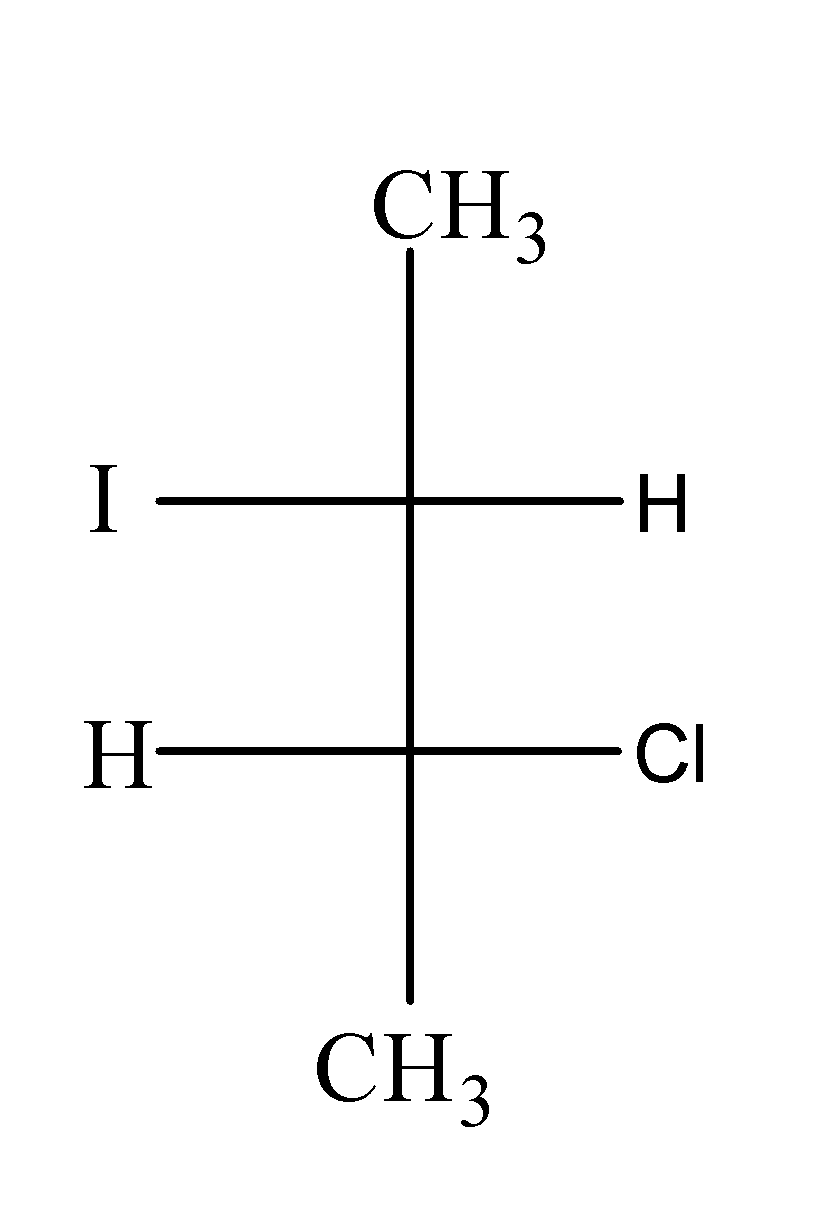
(c)
(d)





Answer
577.8k+ views
Hint: The ${{S}_{N}}2$ is a nucleophilic substitutions reaction i.e. involves the nucleophile and the, substitution of functional group in the molecule with the nucleophile. And in ${{S}_{N}}2$ reactions, the nucleophile attack and the departure of the leaving group occurs simultaneously and there is inversion of the configuration i.e. the position of the nucleophile is opposite to the leaving group. Now, identify the product.
Complete answer:
All the reactions occurring in organic chemistry follow a particular reaction mechanism i.e., it follows a step by step sequence in which the reaction takes place and the way reactants follow and join together after the formation of either carbocation or through the transition states and how they are transformed into the products.
Similarly, in the same way, ${{S}_{N}}1$ and ${{S}_{N}}2$are two such reaction mechanisms. Here, in this S is for substitution i.e. those chemical reactions in which one functional group is replaced by the other and N stands for the nucleophilic because the nucleophile ( nucleus loving) is being substituted with the other one.
${{S}_{N}}1$ reactions occur in two steps . In the first step the formation of carbocation takes and is the slowest step and is the rate determining step and in the second step, nucleophile attacks the (which can donate electrons) carbocation and is a very fast reaction. So , thus, in these reactions rate of reaction depends only on the slowest rate determining step i.e. the alkyl halide and thus, are unimolecular reactions.
On the other hand, in ${{S}_{N}}2$reactions, the reaction occurs in a single step through the formation of the transition state and the reactions depend on both the reactants involved and thus, are bimolecular reactions. In this, the incoming nucleophile attacks the molecule from the back side i.e. the opposite side from which the leaving group leaves and thus, results in the inversion of configuration.
Now considering the statement;
When $C{{H}_{3}}CH(Br)CH(Cl)C{{H}_{3}}$ is treated with NaI in acetone , it undergoes ${{S}_{N}}2$and results in the formation of $C{{H}_{3}}CH(I)CH(Cl)C{{H}_{3}}$as;
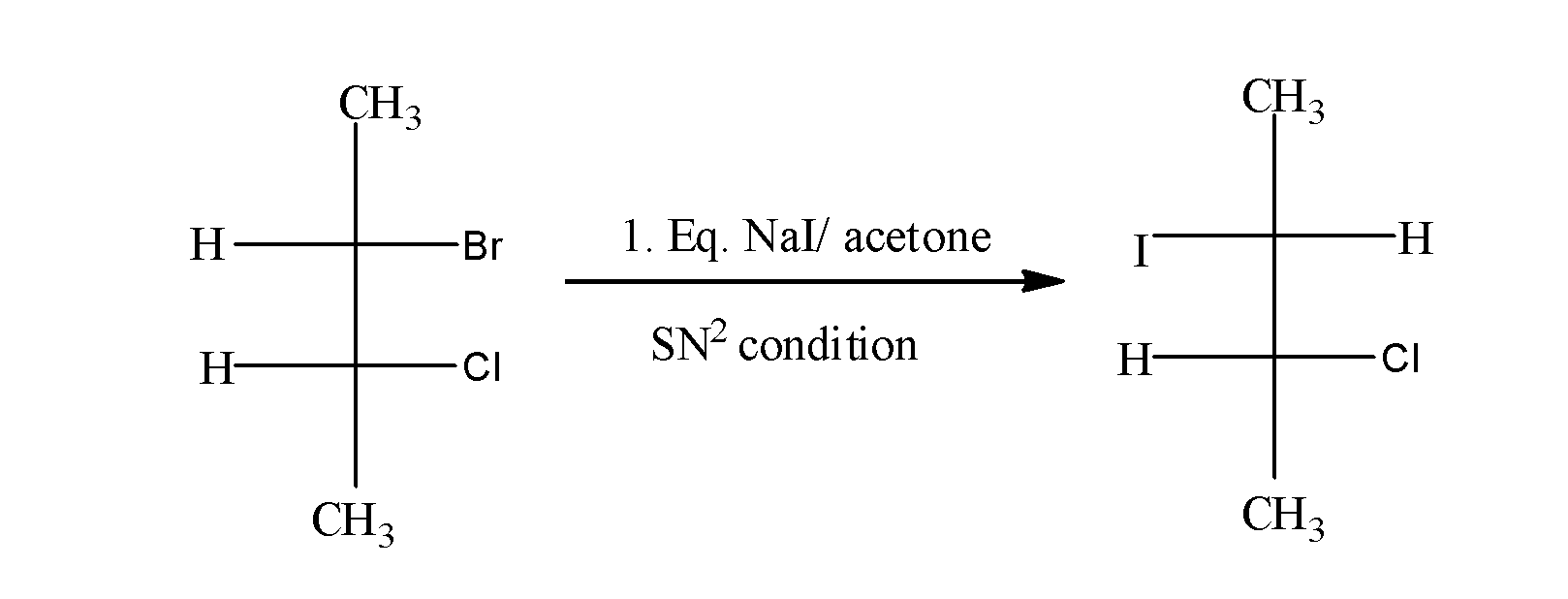
In this reaction, I attack the carbon from the opposite side from where the Br is leaving and results into the inversion of configuration.
Hence, option (c) is correct.
Note:
In ${{S}_{N}}1$, the nucleophile attacks only after the leaving group leaves the molecule and is thus a two-step reaction. On the other hand, in ${{S}_{N}}2$, the nucleophile attacks the molecule from the back side of the molecule as the leaving group is leaving from the front side and is thus, is a single step reaction and is, therefore also called as the concerted mechanism.
Complete answer:
All the reactions occurring in organic chemistry follow a particular reaction mechanism i.e., it follows a step by step sequence in which the reaction takes place and the way reactants follow and join together after the formation of either carbocation or through the transition states and how they are transformed into the products.
Similarly, in the same way, ${{S}_{N}}1$ and ${{S}_{N}}2$are two such reaction mechanisms. Here, in this S is for substitution i.e. those chemical reactions in which one functional group is replaced by the other and N stands for the nucleophilic because the nucleophile ( nucleus loving) is being substituted with the other one.
${{S}_{N}}1$ reactions occur in two steps . In the first step the formation of carbocation takes and is the slowest step and is the rate determining step and in the second step, nucleophile attacks the (which can donate electrons) carbocation and is a very fast reaction. So , thus, in these reactions rate of reaction depends only on the slowest rate determining step i.e. the alkyl halide and thus, are unimolecular reactions.
On the other hand, in ${{S}_{N}}2$reactions, the reaction occurs in a single step through the formation of the transition state and the reactions depend on both the reactants involved and thus, are bimolecular reactions. In this, the incoming nucleophile attacks the molecule from the back side i.e. the opposite side from which the leaving group leaves and thus, results in the inversion of configuration.
Now considering the statement;
When $C{{H}_{3}}CH(Br)CH(Cl)C{{H}_{3}}$ is treated with NaI in acetone , it undergoes ${{S}_{N}}2$and results in the formation of $C{{H}_{3}}CH(I)CH(Cl)C{{H}_{3}}$as;

In this reaction, I attack the carbon from the opposite side from where the Br is leaving and results into the inversion of configuration.
Hence, option (c) is correct.
Note:
In ${{S}_{N}}1$, the nucleophile attacks only after the leaving group leaves the molecule and is thus a two-step reaction. On the other hand, in ${{S}_{N}}2$, the nucleophile attacks the molecule from the back side of the molecule as the leaving group is leaving from the front side and is thus, is a single step reaction and is, therefore also called as the concerted mechanism.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




