
You are given a hammer, a battery, a bulb, wires and a switch
(a) How could you use them to distinguish between samples of metals and Non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and nonmetals.
Answer
504.3k+ views
Hint: We know that Metals are malleable and ductile whereas non-metals are brittle. Also we know that metals are good conductors of heat and electricity while non-metals are poor or bad conductors. By the given materials we can distinguish between metals and non-metals by manipulating these properties of both the types of materials.
Complete answer:
(a) If the substance can be hammered and made into sheets then it is metal. If it is broken into tiny pieces when hit by the hammer then it is non-metal since nonmetals are brittle.
Another way of distinguishing is by using the bulb, battery and the switch. Set up the arrangement as shown in figure.
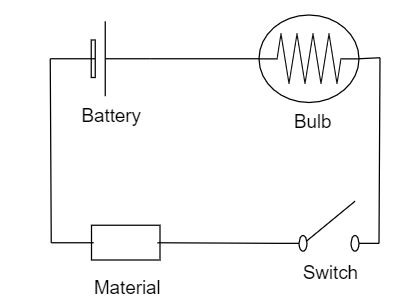
Place the given substance in the marked position, if the bulb starts glowing then the substance is a Metal, because metals are good conductors of electricity. Otherwise we can say that it is non-metal.
(b) The above methods can be used to identify metals and non-metals generally. But there are exceptions in using these methods. For example Sodium and potassium are metals but they are not malleable and are quite brittle. Graphite is an allotrope of carbon and thus is a non-metal, but it is a good conductor of electricity.
Note:
Many chemical tests can also be used to distinguish metals and non-metals. Metals will react with water to form hydroxide and hydrogen gas whereas Non-metals do not react with water. Metals react with dilute acids to form salt and Hydrogen gas whereas Non-metals do not react with dilute acid as they cannot displace hydrogen.
Complete answer:
(a) If the substance can be hammered and made into sheets then it is metal. If it is broken into tiny pieces when hit by the hammer then it is non-metal since nonmetals are brittle.
Another way of distinguishing is by using the bulb, battery and the switch. Set up the arrangement as shown in figure.

Place the given substance in the marked position, if the bulb starts glowing then the substance is a Metal, because metals are good conductors of electricity. Otherwise we can say that it is non-metal.
(b) The above methods can be used to identify metals and non-metals generally. But there are exceptions in using these methods. For example Sodium and potassium are metals but they are not malleable and are quite brittle. Graphite is an allotrope of carbon and thus is a non-metal, but it is a good conductor of electricity.
Note:
Many chemical tests can also be used to distinguish metals and non-metals. Metals will react with water to form hydroxide and hydrogen gas whereas Non-metals do not react with water. Metals react with dilute acids to form salt and Hydrogen gas whereas Non-metals do not react with dilute acid as they cannot displace hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




