
Xenon is a noble gas but it forms compounds, why? Draw the structure of any two compounds.
Answer
581.7k+ views
Hint: Xenon is a noble gas having electronic configuration \[\left[ {{\mathbf{Kr}}} \right]{\text{ }}{\mathbf{4}}{{\mathbf{d}}^{{\mathbf{10}}}}{\mathbf{5}}{{\mathbf{s}}^{\mathbf{2}}}{\mathbf{5}}{{\mathbf{p}}^{\mathbf{6}}}^{}\]and their valence shell orbitals completely filled and, therefore, react with a few elements only under certain condition. Therefore, it is known as Noble gas.
Complete answer:
Let us discuss why noble gases are least reactive. Their inertness to chemical reactivity is due to the following reasons.
The noble gases except helium (\[1{s^2}\]) have completely filled \[n{s^2}n{p^6}\]electronic configuration in their valence shell.
They have high ionization enthalpy means it is very difficult to remove electron from valence shell and they have more positive electron gain enthalpy means they don't accept the electron.
Now we will know the reason why Xenon forms compounds instead of being noble gas.
In March 1962, Neil Bartlett, observed the reaction of a noble gas. First he prepared a red compound which is formulated as \[{{\mathbf{O}}_{{\mathbf{2}} }}^+{\mathbf{Pt}}{{\mathbf{F}}_{\mathbf{6}}}^ - .\]He observed that first ionisation enthalpy of molecular oxygen is similar to Xenon. Then he tried to make similar compound of xenon and he got success to prepare a compound \[{\mathbf{X}}{{\mathbf{e}}^ + }{\mathbf{Pt}}{{\mathbf{F}}_{{\mathbf{6}} }}^-\] by mixing \[{\mathbf{Pt}}{{\mathbf{F}}_{\mathbf{6}}}\] and Xenon.
After this a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen have been formed.
Now Let us see the compounds which are formed by Xenon.
\[{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{2}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{4}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{6}}},{\text{ }}{\mathbf{XeO}}{{\mathbf{F}}_{\mathbf{4}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{O}}_{\mathbf{3}}}\]
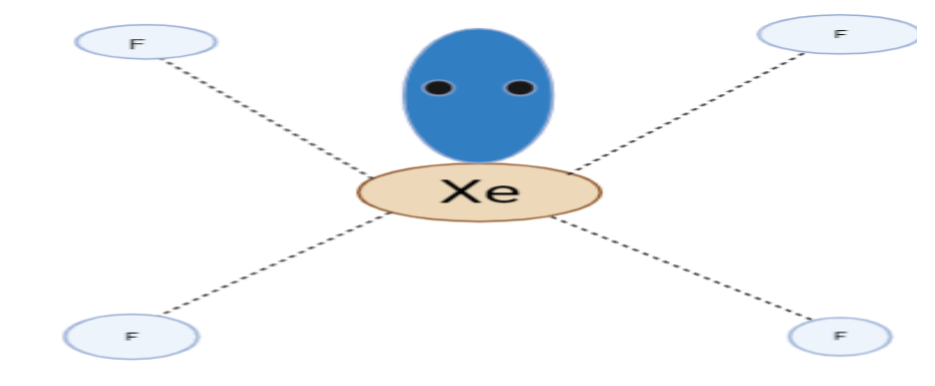
Structure of $XeF_4$
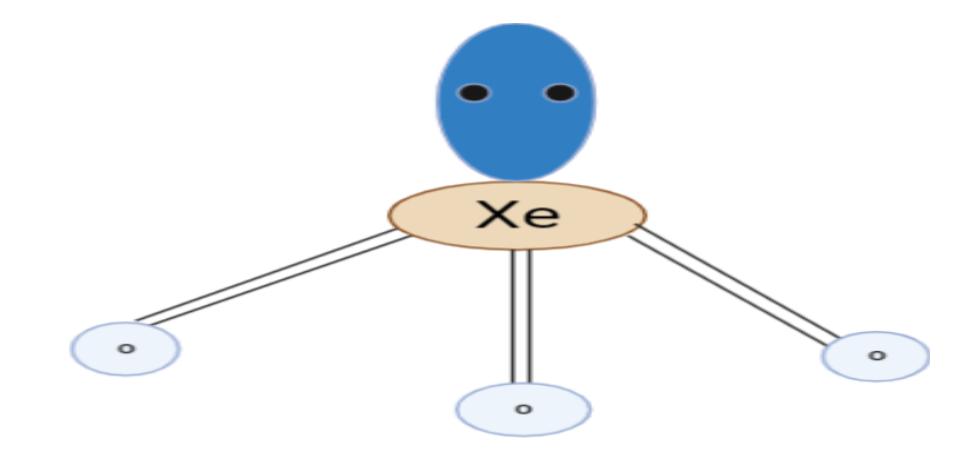
Structure of $XeO_3$
Note:
The study of noble gases and its behaviors is another topic which has to be studied in detail. This helps us understand its uses in daily life and how some of them could be used to produce new compounds.
Complete answer:
Let us discuss why noble gases are least reactive. Their inertness to chemical reactivity is due to the following reasons.
The noble gases except helium (\[1{s^2}\]) have completely filled \[n{s^2}n{p^6}\]electronic configuration in their valence shell.
They have high ionization enthalpy means it is very difficult to remove electron from valence shell and they have more positive electron gain enthalpy means they don't accept the electron.
Now we will know the reason why Xenon forms compounds instead of being noble gas.
In March 1962, Neil Bartlett, observed the reaction of a noble gas. First he prepared a red compound which is formulated as \[{{\mathbf{O}}_{{\mathbf{2}} }}^+{\mathbf{Pt}}{{\mathbf{F}}_{\mathbf{6}}}^ - .\]He observed that first ionisation enthalpy of molecular oxygen is similar to Xenon. Then he tried to make similar compound of xenon and he got success to prepare a compound \[{\mathbf{X}}{{\mathbf{e}}^ + }{\mathbf{Pt}}{{\mathbf{F}}_{{\mathbf{6}} }}^-\] by mixing \[{\mathbf{Pt}}{{\mathbf{F}}_{\mathbf{6}}}\] and Xenon.
After this a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen have been formed.
Now Let us see the compounds which are formed by Xenon.
\[{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{2}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{4}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{F}}_{\mathbf{6}}},{\text{ }}{\mathbf{XeO}}{{\mathbf{F}}_{\mathbf{4}}},{\text{ }}{\mathbf{Xe}}{{\mathbf{O}}_{\mathbf{3}}}\]

Structure of $XeF_4$

Structure of $XeO_3$
Note:
The study of noble gases and its behaviors is another topic which has to be studied in detail. This helps us understand its uses in daily life and how some of them could be used to produce new compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




