
$ X $ is mixed with a mixture of phenol and aniline in an acidic medium. The product obtained is:
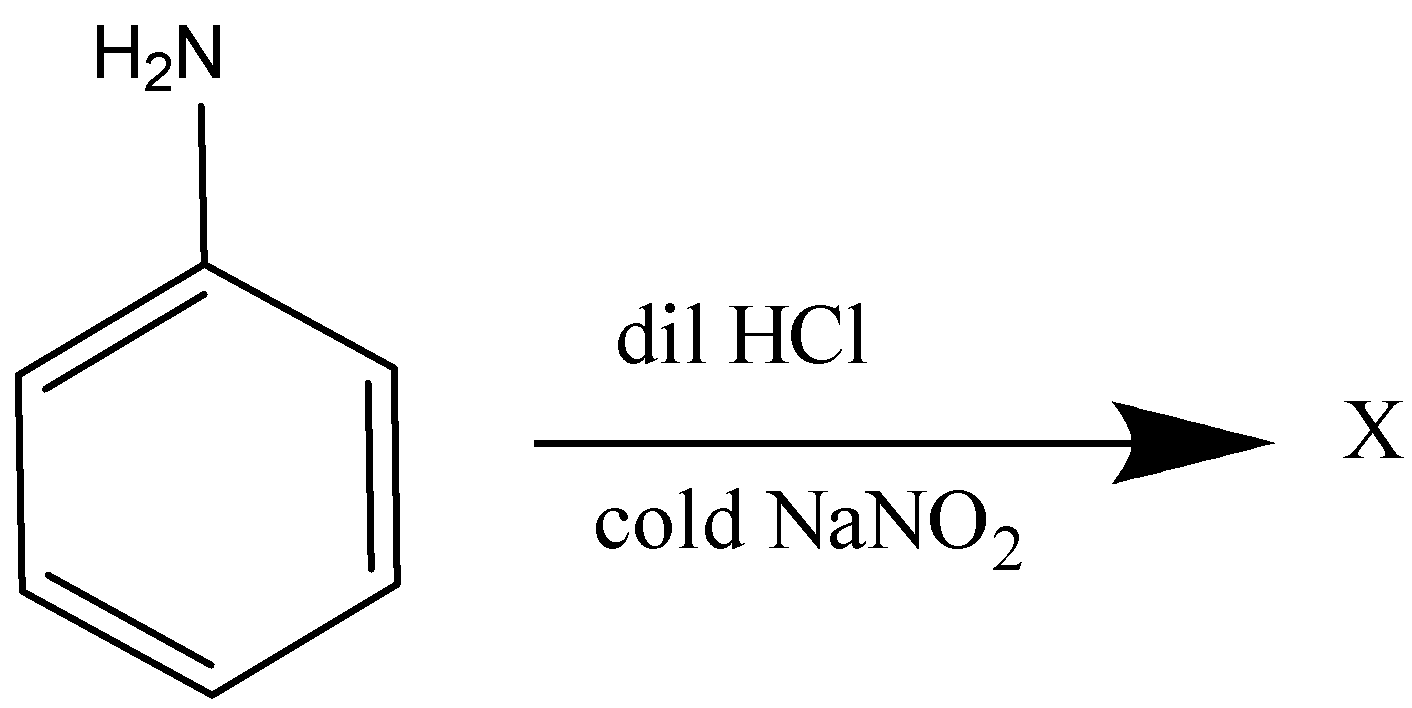
A:
B:
C:
D:





Answer
541.8k+ views
Hint : The reagents involved in the above reaction are the diazotization reagents. Aniline will react in the presence of dilute hydrochloric acid and cold sodium nitrite to form the diazonium salt. Diazotization reaction is the one in which the primary aromatic amines are converted into diazonium salt of the aromatic amine.
Complete Step By Step Answer:
We know that the compounds in which the amino or substituted amino group is attached directly to the benzene ring are known as aromatic amines.
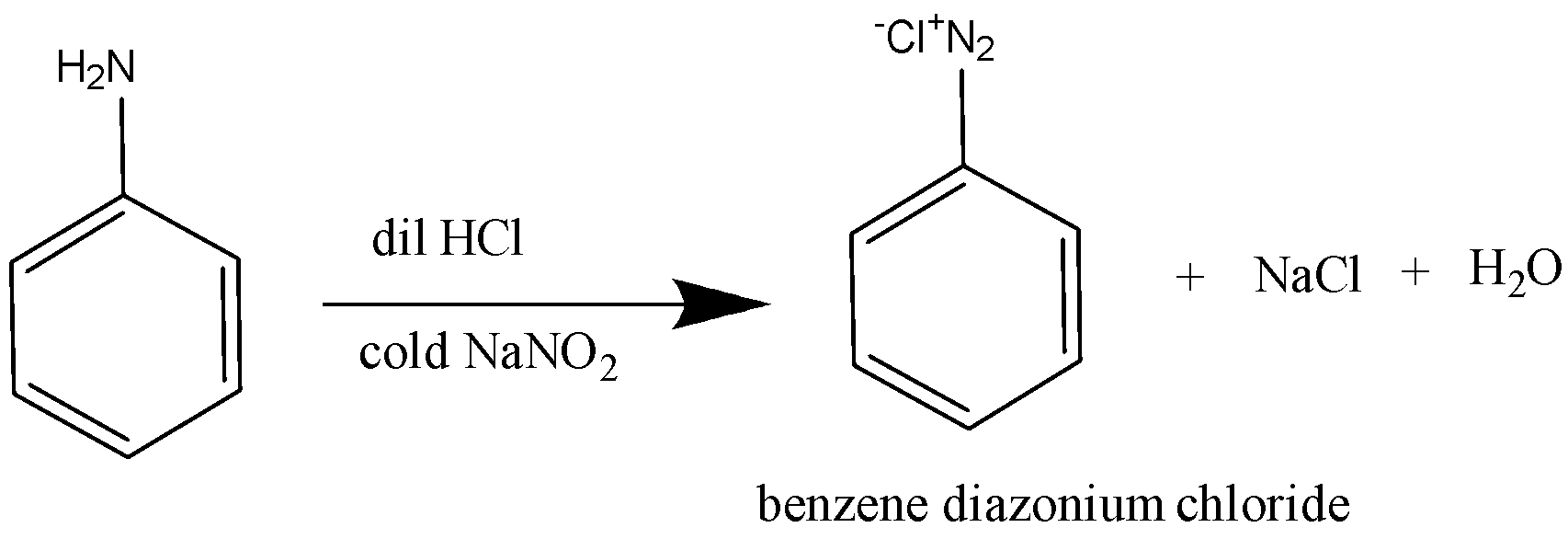
So, the reaction takes place as:
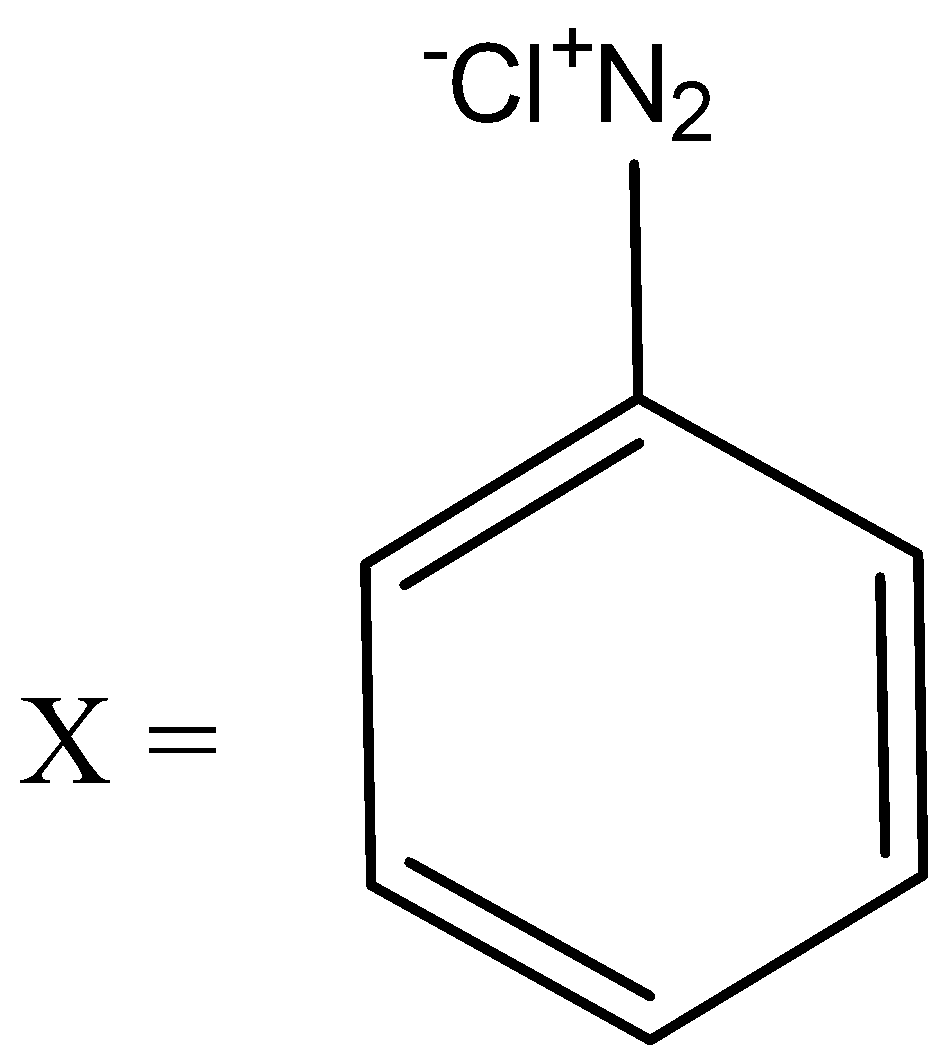
So here $ X $ formed is the diazonium salt which is:
Now it is given that the compound $ X $ is mixed with phenol and aniline in an acidic medium, so let us see how the reaction proceeds.
So, we know that phenol has an oxygen atom in it and aniline has a nitrogen atom present in it so here the diazonium salt will react with aniline as we know that the nitrogen present in it contains lone pair which will be delocalized in the ring and can easily donate electrons to the $ {N_2}^ + $ group of the diazonium salt which is electron deficient and needs electrons. On the other hand, we know that oxygen is more electronegative than nitrogen so it will not donate its electrons easily.
Hence the reaction will proceed as:
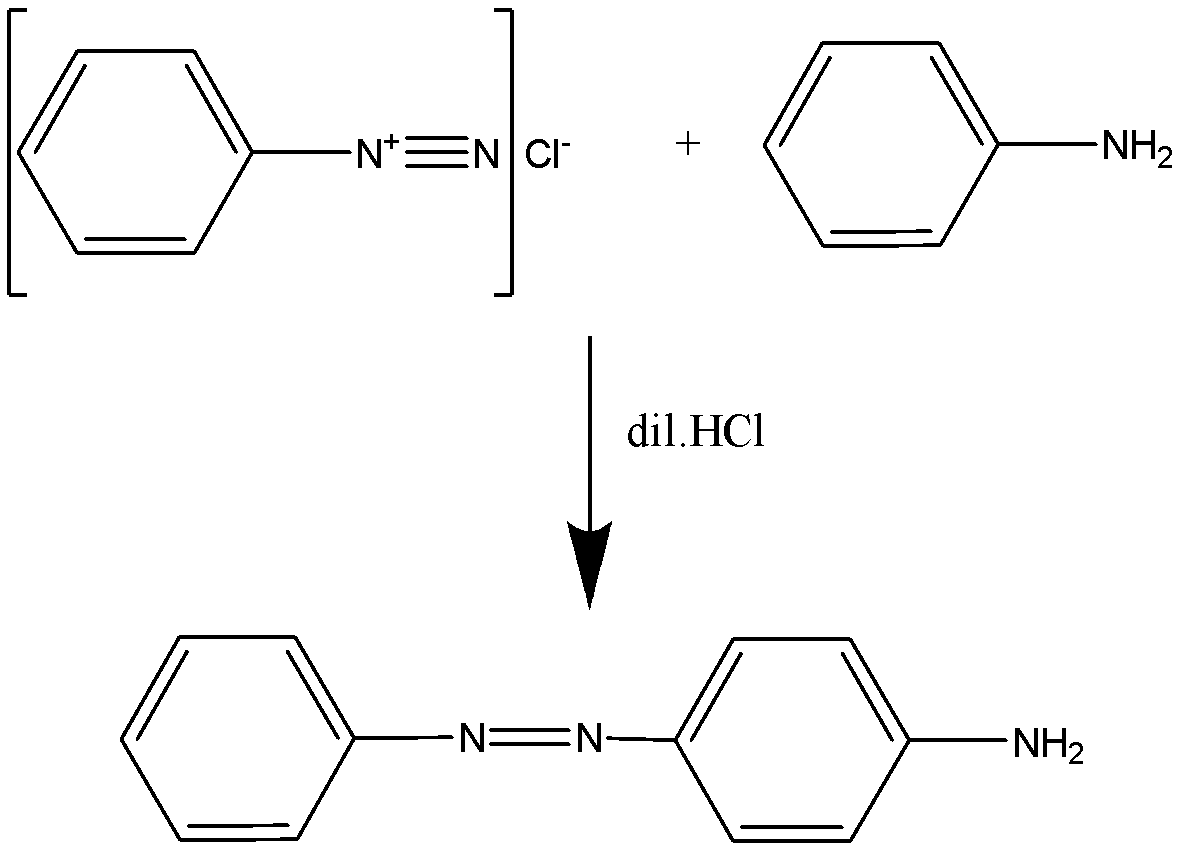
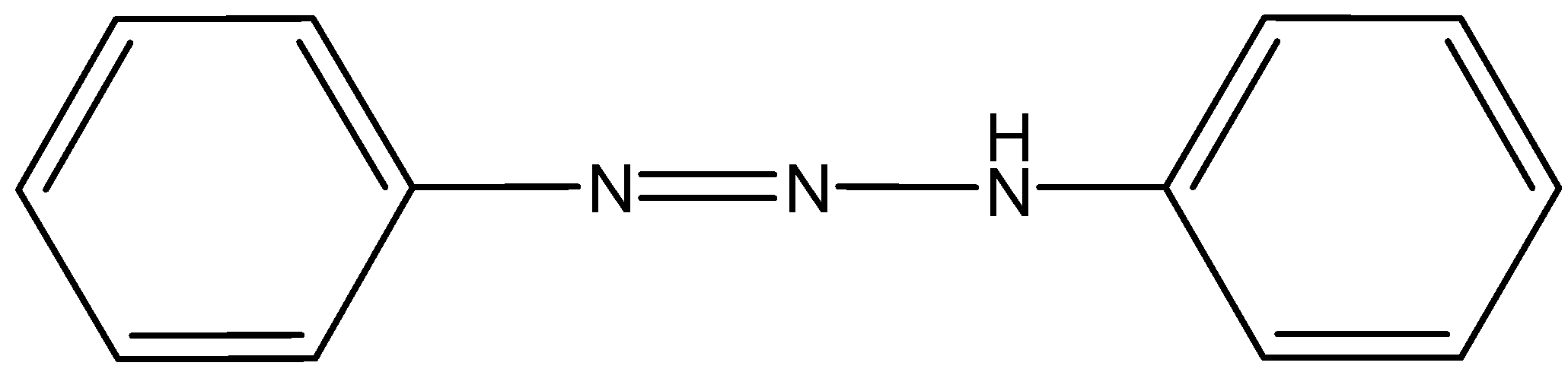
The compound formed is known as $ p - $ amino azobenzene.
Therefore, the correct answer is Option A.
Note :
The temperature required to convert aromatic amine into diazonium salt is around $ 273K - 278K $ Also this reaction is called the coupling reaction. The $ p - $ amino azobenzene.
Formed is a yellow – colored dye. The coupling reaction is also used to prepare the methyl orange dye which is generally used in acid – base titration.
Complete Step By Step Answer:
We know that the compounds in which the amino or substituted amino group is attached directly to the benzene ring are known as aromatic amines.
So, the reaction takes place as:

So here $ X $ formed is the diazonium salt which is:

Now it is given that the compound $ X $ is mixed with phenol and aniline in an acidic medium, so let us see how the reaction proceeds.
So, we know that phenol has an oxygen atom in it and aniline has a nitrogen atom present in it so here the diazonium salt will react with aniline as we know that the nitrogen present in it contains lone pair which will be delocalized in the ring and can easily donate electrons to the $ {N_2}^ + $ group of the diazonium salt which is electron deficient and needs electrons. On the other hand, we know that oxygen is more electronegative than nitrogen so it will not donate its electrons easily.
Hence the reaction will proceed as:

The compound formed is known as $ p - $ amino azobenzene.
Therefore, the correct answer is Option A.
Note :
The temperature required to convert aromatic amine into diazonium salt is around $ 273K - 278K $ Also this reaction is called the coupling reaction. The $ p - $ amino azobenzene.
Formed is a yellow – colored dye. The coupling reaction is also used to prepare the methyl orange dye which is generally used in acid – base titration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




