
What is $ X $ in the following reaction?
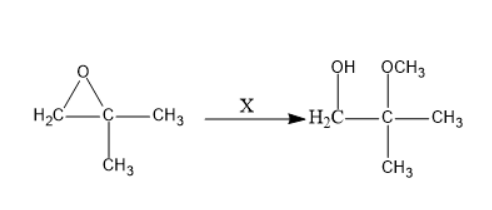
(A) $ C{H_3}OH,{H_2}S{O_4} $
(B) $ C{H_3}OH,C{H_3}{O^ - }N{a^ + } $
(C) $ {H_2}O/{H_2}S{O_4} $ followed by $ C{H_3}OH $
(D) $ C{H_3}MgBr/ether $ followed by $ {H_3}{O^ + } $

Answer
481.8k+ views
Hint: Epoxides are cyclic ethers, in which the oxygen atoms and alkyl groups were arranged in a cyclic form. When the epoxides are treated with sulphuric acid in presence of methanol, the bond between the oxygen and adjacent carbon will break leading to the formation of corresponding alcohol.
Complete Step By Step Answer:
Chemical compounds are classified into functional groups based on the groups or molecules present in the compound. When an oxygen atom is present in between the two alkyl groups. Then that compound can be known as ether. When ether is arranged in a cyclic form, then those compounds were known as epoxides.
Given that a cyclic ether is treated with a chemical reagent $ X $ it leads to the cleavage of oxygen and carbon bonds and forms corresponding alcohol.
When epoxides are treated with methanol in presence of a strong acid like sulphuric acid. The hydrogen atom can be lost from the methanol that generates a nucleophile. Due to the electronegativity of oxygen, it attracts the electron from the adjacent tertiary carbon. Now, these nucleophile attacks on the epoxide lead to the formation of the below product.
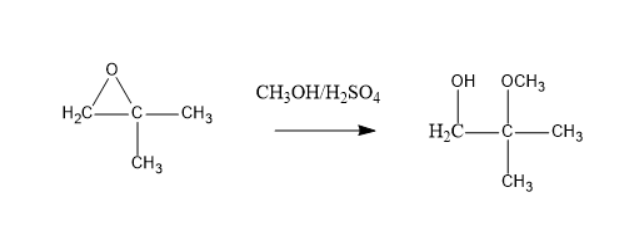
Thus, $ X $ is $ C{H_3}OH,{H_2}S{O_4} $
Option A is the correct one.
Note:
The oxygen atom in epoxide attracts the electrons from the adjacent tertiary carbon atom only, but not the adjacent primary carbon atom. As the tertiary carbocation is more stable than the primary carbocation. Finally, based on the cleavage of carbon-oxygen the alcohol was formed.
Complete Step By Step Answer:
Chemical compounds are classified into functional groups based on the groups or molecules present in the compound. When an oxygen atom is present in between the two alkyl groups. Then that compound can be known as ether. When ether is arranged in a cyclic form, then those compounds were known as epoxides.
Given that a cyclic ether is treated with a chemical reagent $ X $ it leads to the cleavage of oxygen and carbon bonds and forms corresponding alcohol.
When epoxides are treated with methanol in presence of a strong acid like sulphuric acid. The hydrogen atom can be lost from the methanol that generates a nucleophile. Due to the electronegativity of oxygen, it attracts the electron from the adjacent tertiary carbon. Now, these nucleophile attacks on the epoxide lead to the formation of the below product.

Thus, $ X $ is $ C{H_3}OH,{H_2}S{O_4} $
Option A is the correct one.
Note:
The oxygen atom in epoxide attracts the electrons from the adjacent tertiary carbon atom only, but not the adjacent primary carbon atom. As the tertiary carbocation is more stable than the primary carbocation. Finally, based on the cleavage of carbon-oxygen the alcohol was formed.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




