
Write two differences between saturated hydrocarbons and unsaturated hydrocarbons.
Answer
596.1k+ views
Hint: A hydrocarbon is a compound which consists entirely of Hydrogen and Carbon. Further these hydrocarbons are divided into two groups depending on the nature of bond present between Hydrogen and Carbon.
Complete step by step answer:
The hydrocarbon in which all the consecutive carbon atoms are linked by single bonds are called Saturated hydrocarbons.
The hydrocarbons in which consecutive carbon atoms are linked by at least one double or triple bonds are called Unsaturated hydrocarbons.
Since the type of bond between carbon atoms in both types is different, certain differences arise in their properties.
These differences are as follows –
Additional Information: Two major types of saturated hydrocarbons are alkanes and cycloalkanes. Alkanes are straight chain saturated hydrocarbons while cycloalkanes are ring shaped saturated hydrocarbons. The melting and boiling points of straight chain alkanes increase with increase in chain length. Cycloalkanes having the same number of carbon atoms as their corresponding straight chain alkanes, show higher melting and boiling points. Alkanes are used as fuels, heating oils and solvents on a large scale.
Unsaturated hydrocarbons containing double bonds are called alkenes and the ones containing triple bonds are called alkynes. Unsaturated hydrocarbons show low melting and boiling points than saturated ones. Artificial ripening of fruits is done with the help of ethylene which is an unsaturated hydrocarbon. Many chemicals are manufactured industrially with alkenes or alkynes as a starting material.
Note: The major difference of single and double bond gives rise to differences in properties of saturated and unsaturated hydrocarbons. Further, properties of these compounds also change with increasing in the number of carbon atoms.
Complete step by step answer:
The hydrocarbon in which all the consecutive carbon atoms are linked by single bonds are called Saturated hydrocarbons.
The hydrocarbons in which consecutive carbon atoms are linked by at least one double or triple bonds are called Unsaturated hydrocarbons.
Since the type of bond between carbon atoms in both types is different, certain differences arise in their properties.
These differences are as follows –
| Differing character | Saturated hydrocarbons | Unsaturated hydrocarbons |
| Type of C-C bond | All single covalent bonds.No pi bonds. | At least one double or triple bond. Have one or two pi bonds. |
| Hybridization of C atom | All in \[{\text{s}}{{\text{p}}^3}\]. | \[{\text{s}}{{\text{p}}^2}{\text{ }}\] , $sp$ |
| Reactivity and Stability | Less reactive.More stable. | More reactive.Less stable. |
| General formula | \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\] | \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\] for alkynes and \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\]for alkenes |
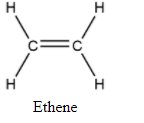
| General structure | 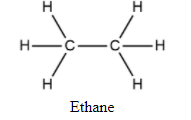
| 
|
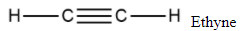
|
Additional Information: Two major types of saturated hydrocarbons are alkanes and cycloalkanes. Alkanes are straight chain saturated hydrocarbons while cycloalkanes are ring shaped saturated hydrocarbons. The melting and boiling points of straight chain alkanes increase with increase in chain length. Cycloalkanes having the same number of carbon atoms as their corresponding straight chain alkanes, show higher melting and boiling points. Alkanes are used as fuels, heating oils and solvents on a large scale.
Unsaturated hydrocarbons containing double bonds are called alkenes and the ones containing triple bonds are called alkynes. Unsaturated hydrocarbons show low melting and boiling points than saturated ones. Artificial ripening of fruits is done with the help of ethylene which is an unsaturated hydrocarbon. Many chemicals are manufactured industrially with alkenes or alkynes as a starting material.
Note: The major difference of single and double bond gives rise to differences in properties of saturated and unsaturated hydrocarbons. Further, properties of these compounds also change with increasing in the number of carbon atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




