
Write the value of p and q for the following conformer of $ 2{\text{ - 3 }} $ dimethyl butane.
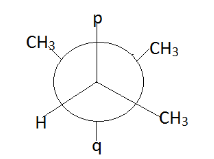
$ 1.{\text{ p = H , q = H}} $
$ 2.{\text{ p = H , q = C}}{{\text{H}}_3} $
$ 3.{\text{ p = C}}{{\text{H}}_3}{\text{ , q = H}} $
$ 4.{\text{ p = C}}{{\text{H}}_3}{\text{ , q = C}}{{\text{H}}_3} $

Answer
507.3k+ views
Hint: Firstly draw the carbon chain structure of $ 2{\text{ - 3 }} $ dimethyl butane. Then we have to make conformers of the given compound. For making conformers we will take reference of $ {C_2} $ and $ 3. $ . Then compare the given conformer with all of them.
Complete answer:
Conformational isomers are those which are formed by just rotating the group about a single bond. For conformers of the compound , it must have a continuous sigma or single bond . In case of double bonds conformers does not exist .Therefore firstly draw the chain structure of given compound,
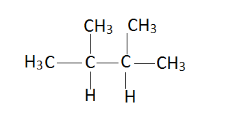
Now give numbering to all carbon atoms.
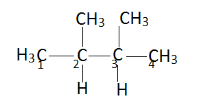
This is $ 2{\text{ - 3 }} $ dimethyl butane. Taking reference of $ {C_2} $ and $ {C_3} $ , we have to rotate the other group around this sigma bond. Therefore the possible conformers will be:
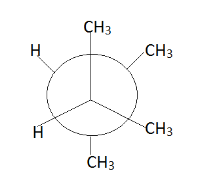
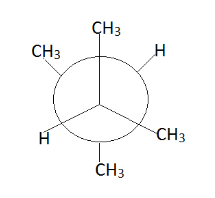
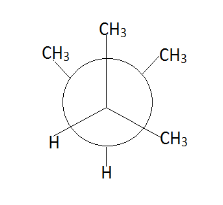
Hence we get the above conformers mainly according to our requirement. Therefore we observe that at place of p there is a $ C{H_3} $ group and at place of q we can replace.
Thus correct option is $ 3. $
Additional Information:
There are other conformers too which may be named as staggered, eclipse and gauche. They have different order of stability .Eclipsed conformation is least stable because of strain .Whereas the staggered conformation is the most stable form among all conformers. Higher stability means less energy. They are the part of stereoisomers.
Note:
There is a condition for conformers that there should be no double bond in continuation of single bond. We can rotate through different angles. The staggered conformation has lowest energy among all conformers while eclipsed has highest energy. This energy is potential energy.
Complete answer:
Conformational isomers are those which are formed by just rotating the group about a single bond. For conformers of the compound , it must have a continuous sigma or single bond . In case of double bonds conformers does not exist .Therefore firstly draw the chain structure of given compound,

Now give numbering to all carbon atoms.

This is $ 2{\text{ - 3 }} $ dimethyl butane. Taking reference of $ {C_2} $ and $ {C_3} $ , we have to rotate the other group around this sigma bond. Therefore the possible conformers will be:



Hence we get the above conformers mainly according to our requirement. Therefore we observe that at place of p there is a $ C{H_3} $ group and at place of q we can replace.
Thus correct option is $ 3. $
Additional Information:
There are other conformers too which may be named as staggered, eclipse and gauche. They have different order of stability .Eclipsed conformation is least stable because of strain .Whereas the staggered conformation is the most stable form among all conformers. Higher stability means less energy. They are the part of stereoisomers.
Note:
There is a condition for conformers that there should be no double bond in continuation of single bond. We can rotate through different angles. The staggered conformation has lowest energy among all conformers while eclipsed has highest energy. This energy is potential energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




