
Write the structure of the product obtained when glucose is oxidised with nitric acid.
Answer
577.5k+ views
Hint: We all know that Glucose is the most abundant carbohydrate and the simplest sugar with a molecular formula ${C_6}{H_{12}}{O_6}$. It is one of the basic necessary sources of energy required in all kinds of organisms. It can exist as a D-glucose and L-glucose configurations.
Complete step by step answer:
As we have learnt till now that glucose occurs in nature in free as well as in combined form and is the most abundant organic compound on Earth. Glucose is an aldohexose that possesses an aldehydic group and it is also the monomer of many bigger carbohydrates like starch, cellulose etc. The structure of glucose can be depicted as:
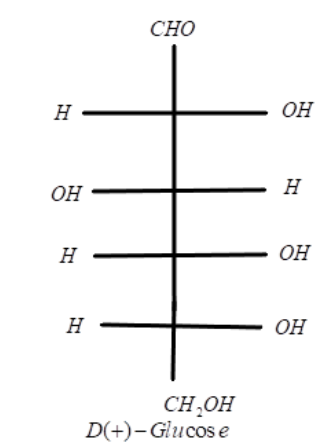
Let us talk about the configuration of glucose as it can exist as glucose D-configuration and glucose L-configuration. When the hydroxyl group on 4th and 5th carbon atom are present on the right hand side of the fischer projection then the configuration is D-glucose and written as D(+)-Glucose, where + sign represents the dextrorotatory nature of the molecule and when the hydroxyl group is present on the left hand side then the configuration results in L-glucose.
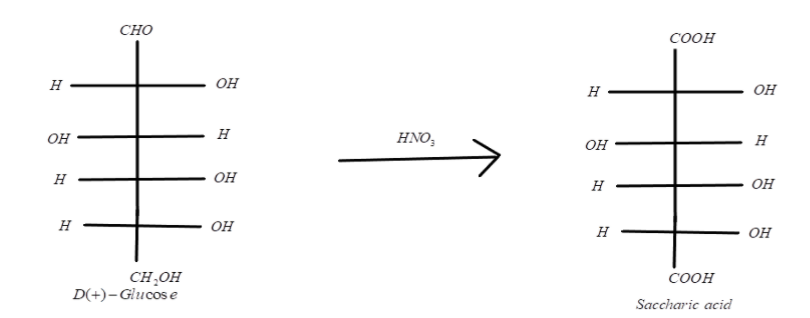
When this D-glucose reacts with $HN{O_3}$ which is a strong oxidising agent, it converts the alcoholic and aldehydic groups present in D-glucose into the carboxylic acid group and results in the formation of D-glucaric acid (Saccharic acid). Gluconic acid reacts with nitric acid; it yields the same product that is Saccharic acid. We can represent it as:
Note:
It is also an interesting fact that Glucose can exist in α and β-anomeric forms as α-D-(+)-Glucose and β-D-(+)-Glucose. These two hemiacetal forms differ only in the configuration of the hydroxyl group at first carbon called anomeric carbon. They are not mirror images of each other and hence they are not enantiomers.
Complete step by step answer:
As we have learnt till now that glucose occurs in nature in free as well as in combined form and is the most abundant organic compound on Earth. Glucose is an aldohexose that possesses an aldehydic group and it is also the monomer of many bigger carbohydrates like starch, cellulose etc. The structure of glucose can be depicted as:

Let us talk about the configuration of glucose as it can exist as glucose D-configuration and glucose L-configuration. When the hydroxyl group on 4th and 5th carbon atom are present on the right hand side of the fischer projection then the configuration is D-glucose and written as D(+)-Glucose, where + sign represents the dextrorotatory nature of the molecule and when the hydroxyl group is present on the left hand side then the configuration results in L-glucose.
When this D-glucose reacts with $HN{O_3}$ which is a strong oxidising agent, it converts the alcoholic and aldehydic groups present in D-glucose into the carboxylic acid group and results in the formation of D-glucaric acid (Saccharic acid). Gluconic acid reacts with nitric acid; it yields the same product that is Saccharic acid. We can represent it as:

Note:
It is also an interesting fact that Glucose can exist in α and β-anomeric forms as α-D-(+)-Glucose and β-D-(+)-Glucose. These two hemiacetal forms differ only in the configuration of the hydroxyl group at first carbon called anomeric carbon. They are not mirror images of each other and hence they are not enantiomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




