
Write the structure of monomer and name the given polymer,
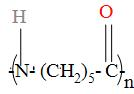
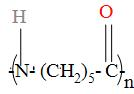
Answer
566.7k+ views
Hint: We can the given polymer consists of amide group (-$\text{CON}{{\text{H}}_{2}}$) and such linkage is also called as amide linkage and such amide linkages are mostly shown by the nylon polymers and are , thus, the condensation polymers. Now identify it.
Complete step by step answer:
First of all, we should know what polymers are actually polymers. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as the polymerization.
The above given polymer is a synthetic condensation polymer which has been developed in the laboratories. Condensation polymers are those polymers which are formed by the molecules which consists of more than one functional group and involves the elimination of water, ammonia etc. and in the given polymer we can see that there are two functional groups i.e. -NH group and -C=O group.
The $\text{-(C}{{\text{H}}_{2}}{{\text{)}}_{5}}-$ group in the polymer represents that the cyclohexane and the two functional groups i.e. -NH and -C=O are attached to it and it loses a water molecule to form this polymer i.e. the two hydrogen atom is lost from the carbon atom of cyclohexane to form the water molecule and now, suppose that the two hydrogen atom are not lost from the polymer, then the resulting structure of monomer is found to be as:
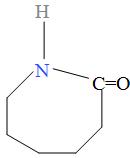
This structure is of the caprolactam and thus, the monomer of the given polymer is caprolactam (monocarboxylic amino acid containing six carbon atoms) and the polymerization of caprolactam with the loss of water results in the formation of polymer Nylon -6 as:
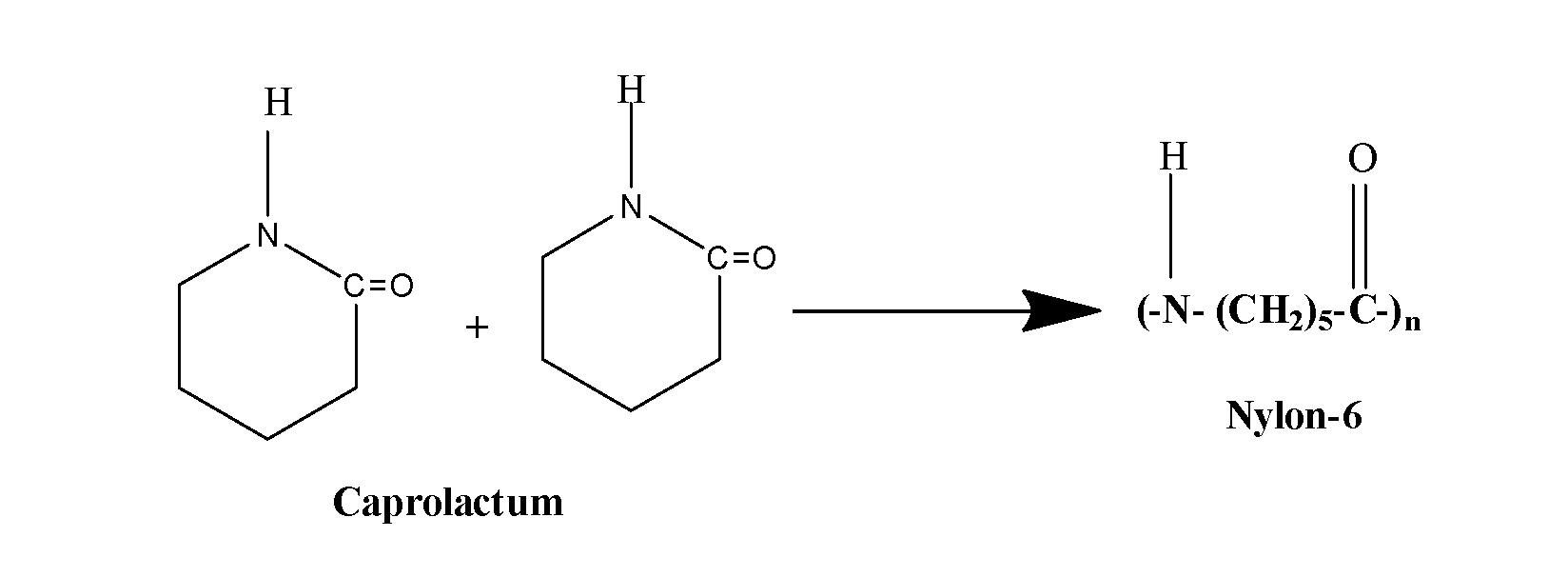
Note: The molten polymer of nylon-6 is forced through the spinner to get the fibres which are cooled by a stream of air and it is used in the manufacture of ropes, fabrics and tyre cords.
Complete step by step answer:
First of all, we should know what polymers are actually polymers. Polymers are the high molecular mass compounds, obtained by joining together a larger number of simple molecules through covalent bonds in a regular manner. And the simple molecules which combine to form a polymer are called the monomers and this process of formation of the polymers is known as the polymerization.
The above given polymer is a synthetic condensation polymer which has been developed in the laboratories. Condensation polymers are those polymers which are formed by the molecules which consists of more than one functional group and involves the elimination of water, ammonia etc. and in the given polymer we can see that there are two functional groups i.e. -NH group and -C=O group.
The $\text{-(C}{{\text{H}}_{2}}{{\text{)}}_{5}}-$ group in the polymer represents that the cyclohexane and the two functional groups i.e. -NH and -C=O are attached to it and it loses a water molecule to form this polymer i.e. the two hydrogen atom is lost from the carbon atom of cyclohexane to form the water molecule and now, suppose that the two hydrogen atom are not lost from the polymer, then the resulting structure of monomer is found to be as:

This structure is of the caprolactam and thus, the monomer of the given polymer is caprolactam (monocarboxylic amino acid containing six carbon atoms) and the polymerization of caprolactam with the loss of water results in the formation of polymer Nylon -6 as:

Note: The molten polymer of nylon-6 is forced through the spinner to get the fibres which are cooled by a stream of air and it is used in the manufacture of ropes, fabrics and tyre cords.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




