
Write the structure of following alkane.
6-Isopropyl -2,3-dimethylnonane.
Answer
566.7k+ views
Hint: Alkanes are saturated hydrocarbons with general formula ${C_n}{H_{2n + 2}}$. Where, C is carbon, H is hydrogen and n is the number of carbon atoms in the compound.
Complete step by step answer:
We will solve this question step by step.
(1) In the first step identify the parent alkane. Here parent alkane is nonane which has 9 Carbon atoms in a straight longest chain. So we will first draw 9 carbon atoms in a straight chain.
C - C - C - C - C - C - C - C - C
(2) Now will start numbering the C-atoms either from left or right. Here we start numbering from left side
$1\;\;\; \;\;\; 2\;\;\;\;\; 3\;\;\;\;\; 4\;\;\;\;\;\; 5\;\;\;\;\;\; 6\;\;\;\;\; 7\;\;\;\;\;\; 8\;\;\;\;\;9$
$C - C - C - C - C - C - C - C - C$
(3) Now put substituents at the respective position. Here substituents are 2 methyl groups and one isopropyl group. Methyl group, $C{H_3}$ is present at 2 and 3 position and isopropyl group, $CH{(C{H_3})_2}$ present at 6 position.
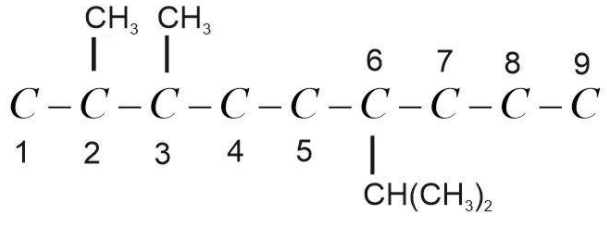
Now balance the remaining valency of C-atoms by attaching H-atoms with it. Valency of C-atom is 4 and the valency of H-atom is 1. It should be satisfied while drawing the structure.
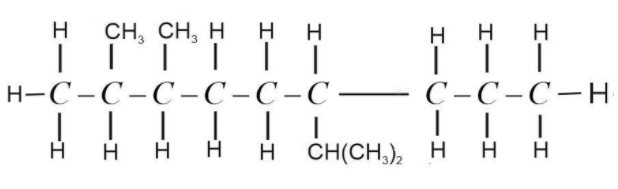
This is the complete structural formula of 6-Isopropyl -2,3-dimethylnonane. It is not always necessary to show the H-atom. We can assume the presence of H-atom and write normal structure formula as,
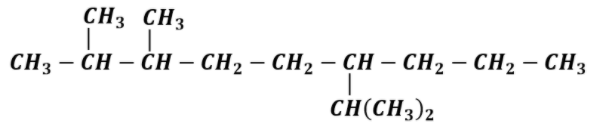
This is the required structure 6-Isopropyl -2,3-dimethylnonane.
Note: While drawing the structure of any compound, see that the valency of each element is satisfied. The valency of elements which are not attached to any molecule is satisfied by attaching H-atoms to them. In case of alkenes and alkynes double and triple bonds respectively are used.
Complete step by step answer:
We will solve this question step by step.
(1) In the first step identify the parent alkane. Here parent alkane is nonane which has 9 Carbon atoms in a straight longest chain. So we will first draw 9 carbon atoms in a straight chain.
C - C - C - C - C - C - C - C - C
(2) Now will start numbering the C-atoms either from left or right. Here we start numbering from left side
$1\;\;\; \;\;\; 2\;\;\;\;\; 3\;\;\;\;\; 4\;\;\;\;\;\; 5\;\;\;\;\;\; 6\;\;\;\;\; 7\;\;\;\;\;\; 8\;\;\;\;\;9$
$C - C - C - C - C - C - C - C - C$
(3) Now put substituents at the respective position. Here substituents are 2 methyl groups and one isopropyl group. Methyl group, $C{H_3}$ is present at 2 and 3 position and isopropyl group, $CH{(C{H_3})_2}$ present at 6 position.
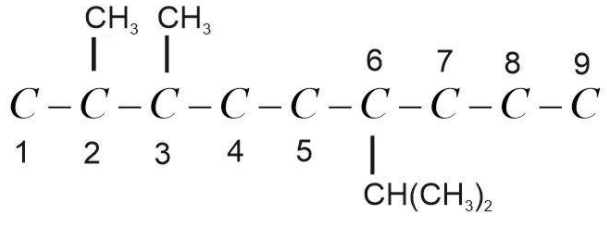
Now balance the remaining valency of C-atoms by attaching H-atoms with it. Valency of C-atom is 4 and the valency of H-atom is 1. It should be satisfied while drawing the structure.

This is the complete structural formula of 6-Isopropyl -2,3-dimethylnonane. It is not always necessary to show the H-atom. We can assume the presence of H-atom and write normal structure formula as,
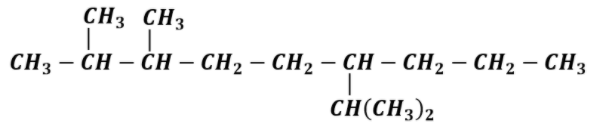
This is the required structure 6-Isopropyl -2,3-dimethylnonane.
Note: While drawing the structure of any compound, see that the valency of each element is satisfied. The valency of elements which are not attached to any molecule is satisfied by attaching H-atoms to them. In case of alkenes and alkynes double and triple bonds respectively are used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




