
Write the structure formula of:
(A) o-Ethylanisole,
(B) p-Nitroaniline,
(C) 2,3 - Dibromo -1 - phenylpentane,
(D) 4-Ethyl-1-fluoro-2-nitrobenzene.
Answer
600.6k+ views
Hint: To answer this question, it is necessary that we should know about drawing structural formulas for organic compounds. We should know that each straight line segment represents a bond, the ends and intersections of the lines are carbon atoms, and the correct number of hydrogen is calculated from the tetra-valency of carbon. Non-bonding valence shell electrons are omitted in these formulas.
Complete step by step answer:
> To answer this question, we will draw the Kekulé Formula of the compounds that are presented in questions. We should know that a structural formula or Kekulé Formula displays the atoms of the molecule in the order they are bonded. It also tells us how the atoms are bonded to one another, for example single, double, and triple covalent bond. Covalent bonds are shown using lines. The number of lines indicates whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful because they explain the properties and structure of the compound which empirical and molecular formulas cannot always represent.
So, now we will draw the structures one by one of each option.
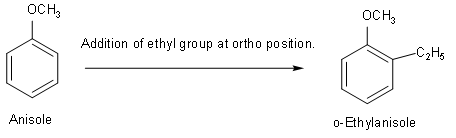
(A) First structure that we will draw is o-Ethylanisole. Its condensed formula is\[{{C}_{9}}{{H}_{12}}O\]. So, first we should know the structure of anisole. And after that we can easily add ethyl groups at ortho position.
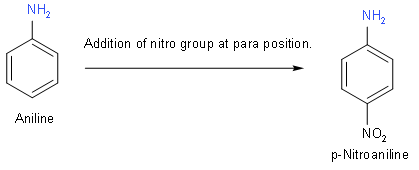
(B) Now, we will draw the structure of p-Nitroaniline. Its formula is \[{{C}_{6}}{{H}_{6}}{{N}_{2}}{{O}_{2}}\]. In this structure we will add a nitro group at para position to aniline.
(C) Now, we will draw the structure of: 2, 3 - Dibromo -1 – phenylpentane. To draw this structure, we will first draw pentane. At second and third position, we will add bromine. And after that at first position, we will add phenol.

(D) Now, we will draw our fourth structure and that is of 4-Ethyl-1-fluoro-2-nitrobenzene. We will first add the nitro group at second place on the benzene ring. Then after this we will add fluorine and ethyl group at first and fourth position respectively.

So, we represented all the structures that are asked in question.
Note:We should know other types of structures as well. We should know that we write condensed formulas to show the order of atoms like a structural formula but we write them in a single line to save space and make it more convenient and faster to write out. We sometimes experience that organic compounds can be complex at times, line-angle formulas are used to write carbon and hydrogen atoms more efficiently by replacing the letters with lines.
Complete step by step answer:
> To answer this question, we will draw the Kekulé Formula of the compounds that are presented in questions. We should know that a structural formula or Kekulé Formula displays the atoms of the molecule in the order they are bonded. It also tells us how the atoms are bonded to one another, for example single, double, and triple covalent bond. Covalent bonds are shown using lines. The number of lines indicates whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful because they explain the properties and structure of the compound which empirical and molecular formulas cannot always represent.
So, now we will draw the structures one by one of each option.
(A) First structure that we will draw is o-Ethylanisole. Its condensed formula is\[{{C}_{9}}{{H}_{12}}O\]. So, first we should know the structure of anisole. And after that we can easily add ethyl groups at ortho position.

(B) Now, we will draw the structure of p-Nitroaniline. Its formula is \[{{C}_{6}}{{H}_{6}}{{N}_{2}}{{O}_{2}}\]. In this structure we will add a nitro group at para position to aniline.

(C) Now, we will draw the structure of: 2, 3 - Dibromo -1 – phenylpentane. To draw this structure, we will first draw pentane. At second and third position, we will add bromine. And after that at first position, we will add phenol.

(D) Now, we will draw our fourth structure and that is of 4-Ethyl-1-fluoro-2-nitrobenzene. We will first add the nitro group at second place on the benzene ring. Then after this we will add fluorine and ethyl group at first and fourth position respectively.

So, we represented all the structures that are asked in question.
Note:We should know other types of structures as well. We should know that we write condensed formulas to show the order of atoms like a structural formula but we write them in a single line to save space and make it more convenient and faster to write out. We sometimes experience that organic compounds can be complex at times, line-angle formulas are used to write carbon and hydrogen atoms more efficiently by replacing the letters with lines.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




