
Write the structural formula of the two isomers of butane.
Answer
579k+ views
Hint: Butane is alkane. It’s general formula is \[{C_n}{H_{2n + 2}}\]. Butane forms structural isomers. These isomers have the same molecular formula but different structural formulae.
Complete step by step answer:
As we know butane is alkane and it has four C – atoms in its molecule
Now put $n = 4$in general formula ${C_n}{H_{2n + 2}}$
${C_4}{H_{2 \times 4 + 2}} = {C_4}{H_{10}}$
Butane shows structural isomerism. The compounds having the same molecular formula but different arrangement of carbon chains within the molecule are called chain isomers or skeletal isomers and this phenomenon is termed as chain isomerism.
Isomers can be drawn as follows.
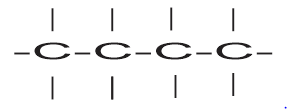
Continuous chain of four C – atoms
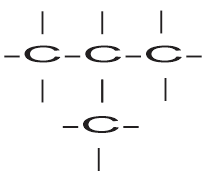
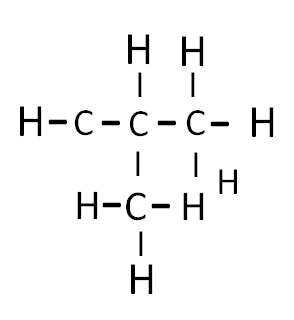
Branched chain with three carbon atoms in long chain
When a carbon atom is present in a continuous chain it is termed as normal butane or n – butane.
When a branch of methyl group present in a secondary carbon of continuous chain, the compound is termed as iso – i.e. isobutane.
$\therefore $There are two isomers of butane are
(i) n – butane (ii) isobutane
Their structures are –
n – butane
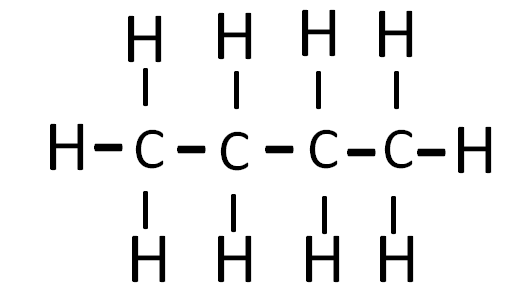
Isobutane
Additional information:
These isomers have different physical properties. Their boiling point decreases as branching increases. With increasing branching, the surface area of the compound decreases so vendor force of attraction also decreases, as a result boiling point decreases.
Note:
While drawing structural formulae make sure that valency of each C – atom should be satisfied.
While drawing different structures of isomers first draw skeletal of isomer and then satisfy valency of each atom by putting H – atoms.
Complete step by step answer:
As we know butane is alkane and it has four C – atoms in its molecule
Now put $n = 4$in general formula ${C_n}{H_{2n + 2}}$
${C_4}{H_{2 \times 4 + 2}} = {C_4}{H_{10}}$
Butane shows structural isomerism. The compounds having the same molecular formula but different arrangement of carbon chains within the molecule are called chain isomers or skeletal isomers and this phenomenon is termed as chain isomerism.
Isomers can be drawn as follows.
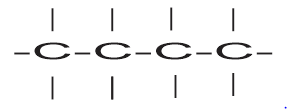
Continuous chain of four C – atoms

Branched chain with three carbon atoms in long chain
When a carbon atom is present in a continuous chain it is termed as normal butane or n – butane.
When a branch of methyl group present in a secondary carbon of continuous chain, the compound is termed as iso – i.e. isobutane.
$\therefore $There are two isomers of butane are
(i) n – butane (ii) isobutane
Their structures are –
n – butane
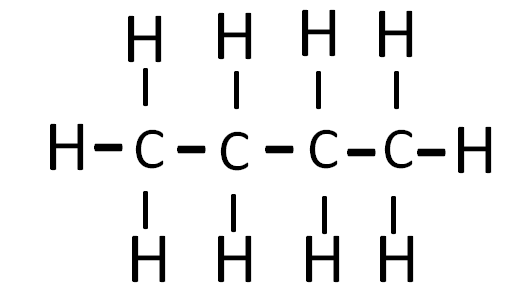
Isobutane

Additional information:
These isomers have different physical properties. Their boiling point decreases as branching increases. With increasing branching, the surface area of the compound decreases so vendor force of attraction also decreases, as a result boiling point decreases.
Note:
While drawing structural formulae make sure that valency of each C – atom should be satisfied.
While drawing different structures of isomers first draw skeletal of isomer and then satisfy valency of each atom by putting H – atoms.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




