
Write the structural formula of ethanol. What happens is it heated with excess of conc.\[{H_2}S{O_4}\] acid at \[443k\]? Write the chemical equation for the reaction, stating the role of conc.\[{H_2}S{O_4}\] acid in this reaction.
Answer
504.6k+ views
Hint: We need to know that the molecular formula of ethyl alcohol is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\]. The molecular weight of ethyl alcohol is \[{\text{46g}}\]. Alcohol is the functional group present in ethyl alcohol. The molecular formula of ethylene is \[{\text{C}}{{\text{H}}_2}{\text{ = C}}{{\text{H}}_{\text{2}}}\]. The molecular weight of ethylene is \[{\text{18g}}\].
Complete answer:
We can draw the structural formula of ethanol is below,
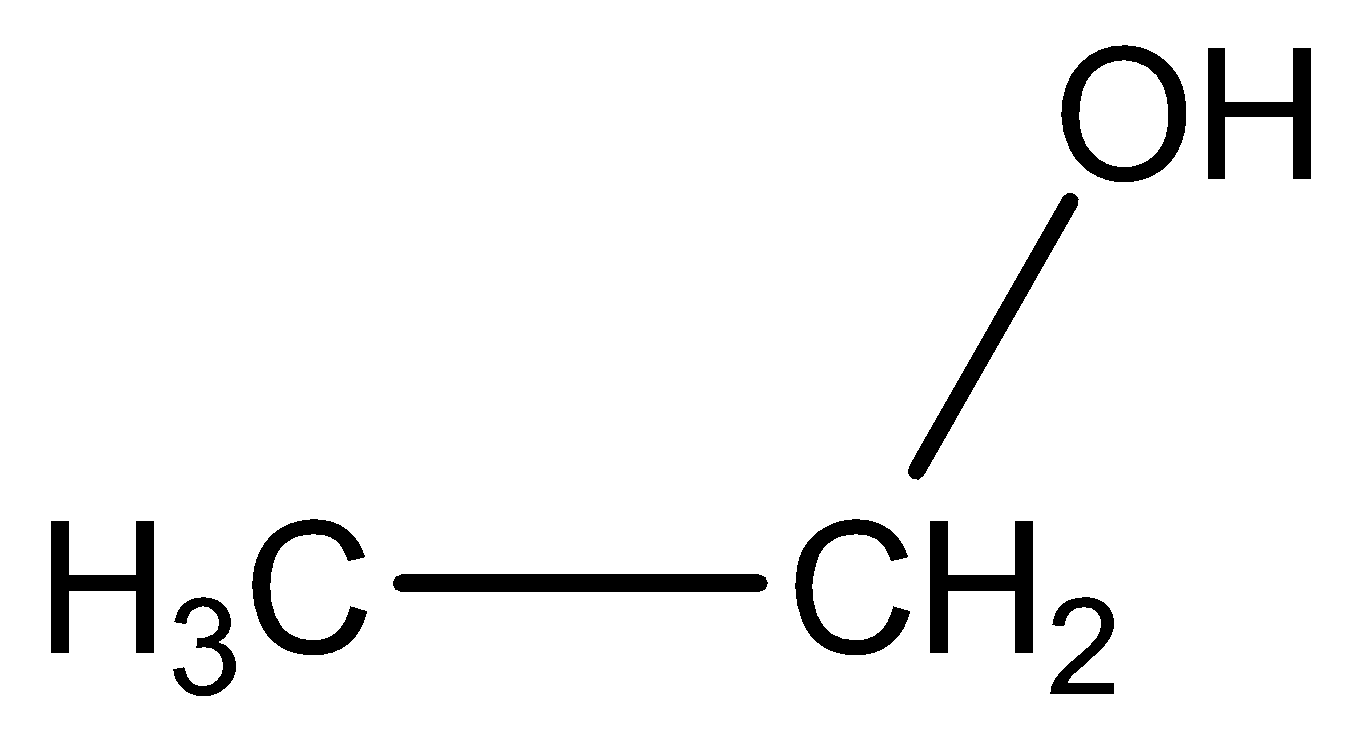
When ethanol is heated with excess of conc.\[{H_2}S{O_4}\] acid at \[443k\] to give a product of ethylene.
The chemical equation for the above discussion is given below,
\[C{H_3}C{H_2}OH + {H_2}S{O_4} \to {\text{C}}{{\text{H}}_2}{\text{ = C}}{{\text{H}}_{\text{2}}}\]
The reaction is given below,
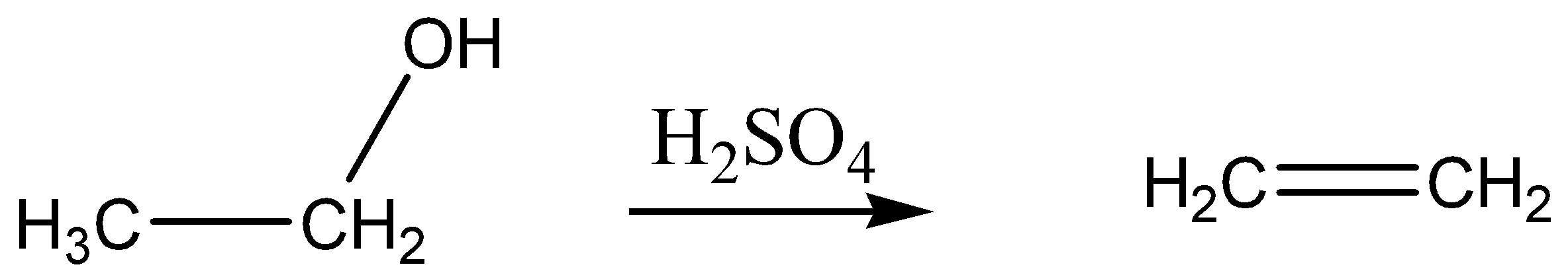
The role of conc.\[{H_2}S{O_4}\] acid in this reaction is a dehydrating agent.
Note:
In organic chemistry oxidation and reduction are important concepts. The oxidation and reduction are used to convert one functional group of molecules to another functional group. One homologous form to another homologous form of hydrocarbon is also possible in oxidation and reduction reaction. In one reaction oxidation followed by reduction reaction or the reduction reaction followed by oxidation reaction is called redox reaction. The oxidation reaction means the addition of oxygen or the removal of hydrogen or the loss of electrons in the reaction from reactant to product. The reduction reaction means the addition of hydrogen or the removal of oxygen or gain of electrons in the reaction from reactant to product.
So, first ethylene is treated with sulphuric acid to form sulfonium intermediate and followed by hydrolysis done means we get ethyl alcohol as a product.
Ethyl alcohol is industrially prepared from ethylene by absorbing in \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\] followed by hydrolysis.
Complete answer:
We can draw the structural formula of ethanol is below,

When ethanol is heated with excess of conc.\[{H_2}S{O_4}\] acid at \[443k\] to give a product of ethylene.
The chemical equation for the above discussion is given below,
\[C{H_3}C{H_2}OH + {H_2}S{O_4} \to {\text{C}}{{\text{H}}_2}{\text{ = C}}{{\text{H}}_{\text{2}}}\]
The reaction is given below,

The role of conc.\[{H_2}S{O_4}\] acid in this reaction is a dehydrating agent.
Note:
In organic chemistry oxidation and reduction are important concepts. The oxidation and reduction are used to convert one functional group of molecules to another functional group. One homologous form to another homologous form of hydrocarbon is also possible in oxidation and reduction reaction. In one reaction oxidation followed by reduction reaction or the reduction reaction followed by oxidation reaction is called redox reaction. The oxidation reaction means the addition of oxygen or the removal of hydrogen or the loss of electrons in the reaction from reactant to product. The reduction reaction means the addition of hydrogen or the removal of oxygen or gain of electrons in the reaction from reactant to product.
So, first ethylene is treated with sulphuric acid to form sulfonium intermediate and followed by hydrolysis done means we get ethyl alcohol as a product.
Ethyl alcohol is industrially prepared from ethylene by absorbing in \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\] followed by hydrolysis.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




