
Write the structural formula and IUPAC name of the given compound with a terminal acid.
Molecular formula: ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}$ .
Answer
589.5k+ views
Hint: The given compound is a terminal acid which means that it contains the carboxyl functional group ${\text{ - COOH}}$ at the end of the chain.
Since the molecular formula is given to be ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}$ and there is a carboxyl ${\text{ - COOH}}$ group present, therefore, the remaining part of the compound has the ${{\text{C}}_3}{{\text{H}}_7}$ group.
Complete step by step answer:
The compound is a carboxylic acid having the formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{ - COOH}}$ .
The ${{\text{C}}_3}{{\text{H}}_7}$ group is propyl group which can also be written as ${\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}$ group.
Therefore, the structural formula of the given compound is
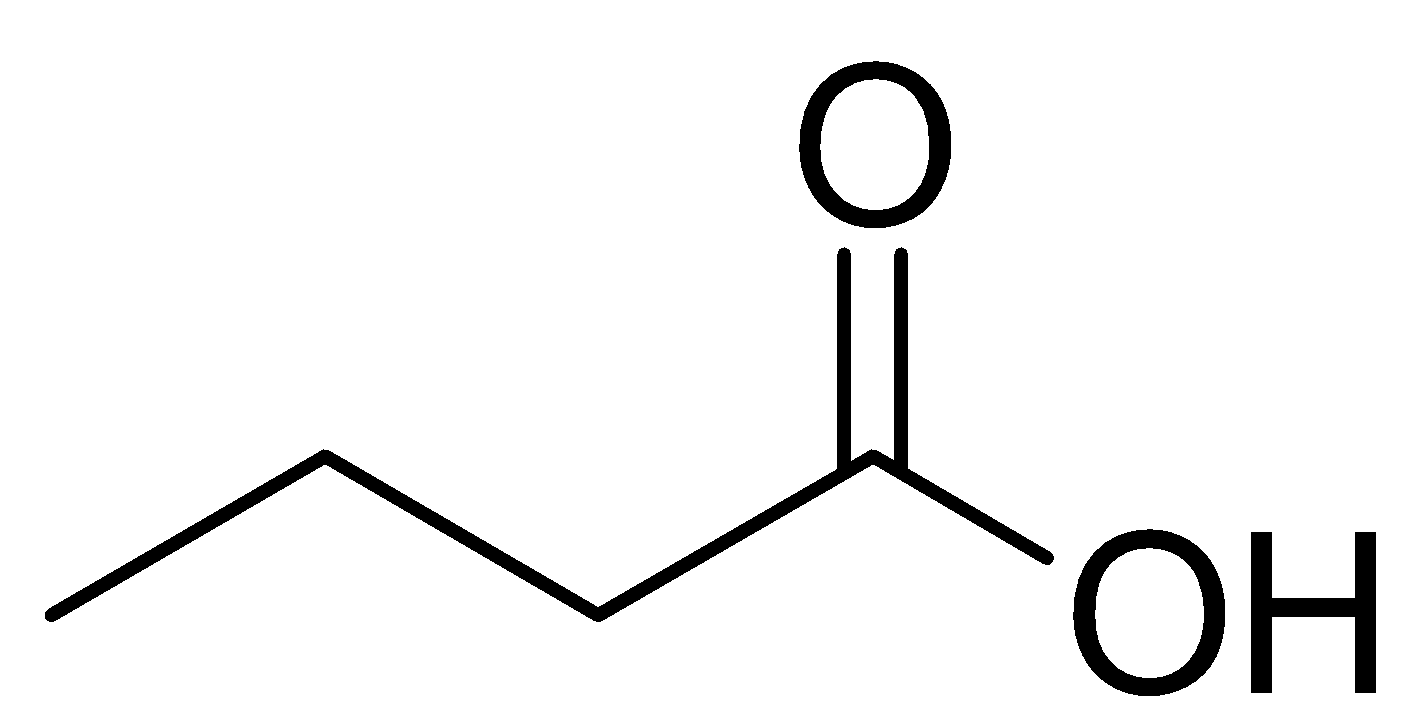
Or,
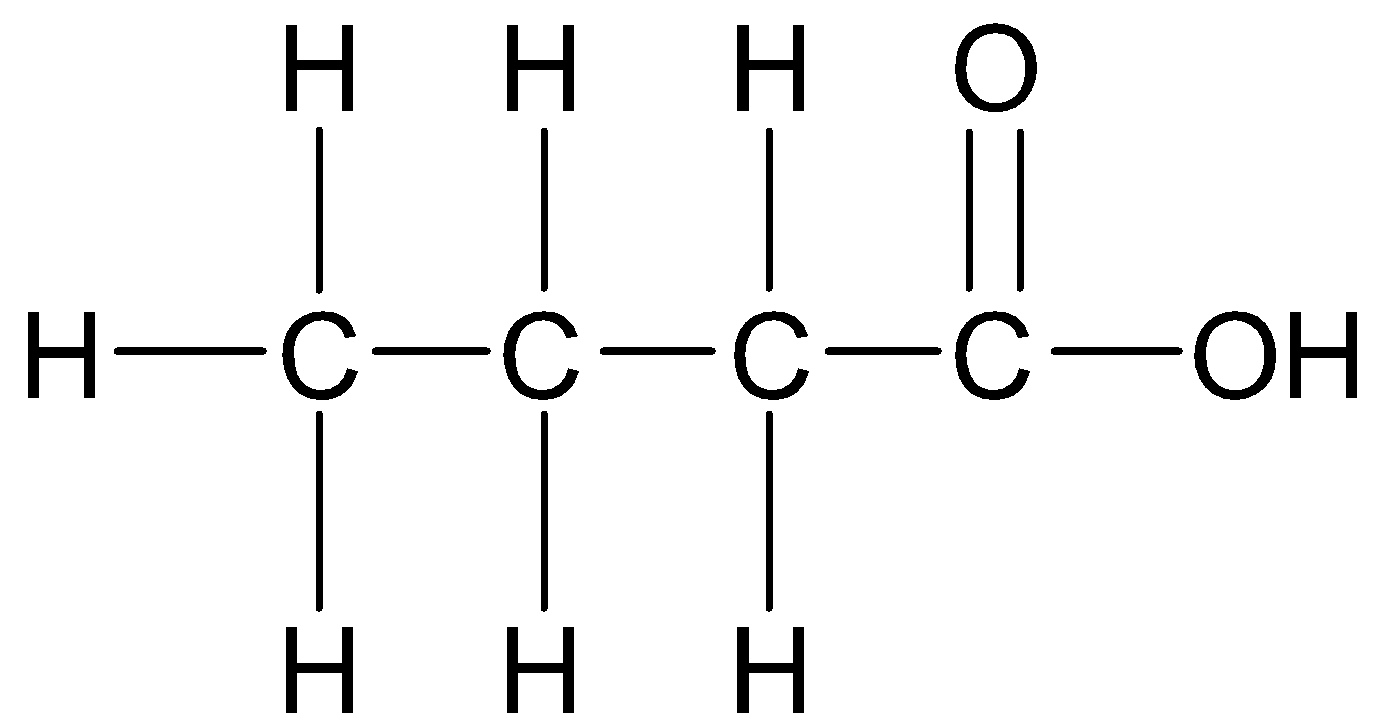
-According to IUPAC nomenclature, if organic compounds contain one principal functional group, then the longest continuous chain of carbon atoms containing the principal functional group is selected.
-If the functional group is such that a carbon atom is already present in it, such as the carboxyl functional group, then the numbering of the carbon chain is done in such a way that this functional group gets the lowest position of 1. While naming the compound, the position 1 is not written with such a group.
-For unsubstituted monocarboxylic acids, the IUPAC name is obtained by changing the suffix ‘ane’ of the corresponding alkane to ‘oic acid’ or in other words, it is ‘alkanoic acid’.
-Now, let us see the structure of the given acid. The carboxyl group is given the position 1 which is omitted while naming. Numbering in this way, we see that there are total 4 carbon atoms involved and so we get:
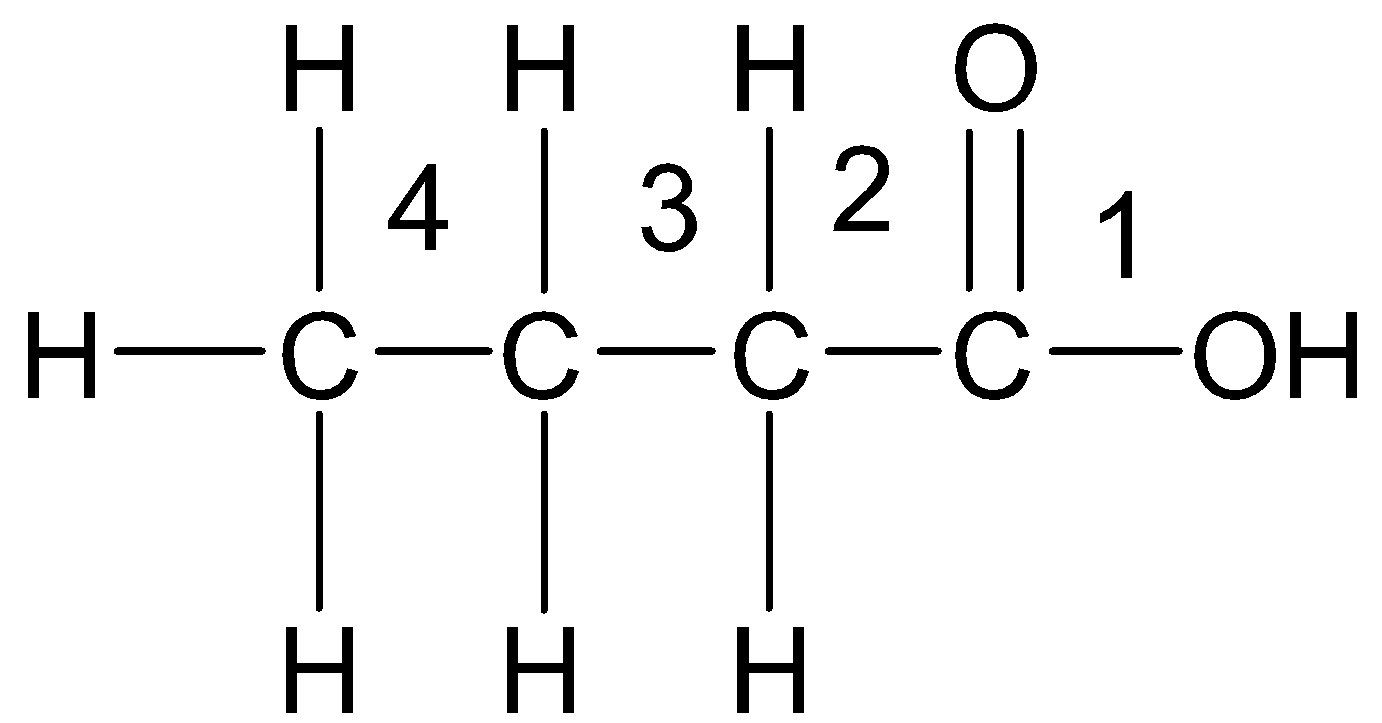
So, the IUPAC name is Butanoic acid.
Note:
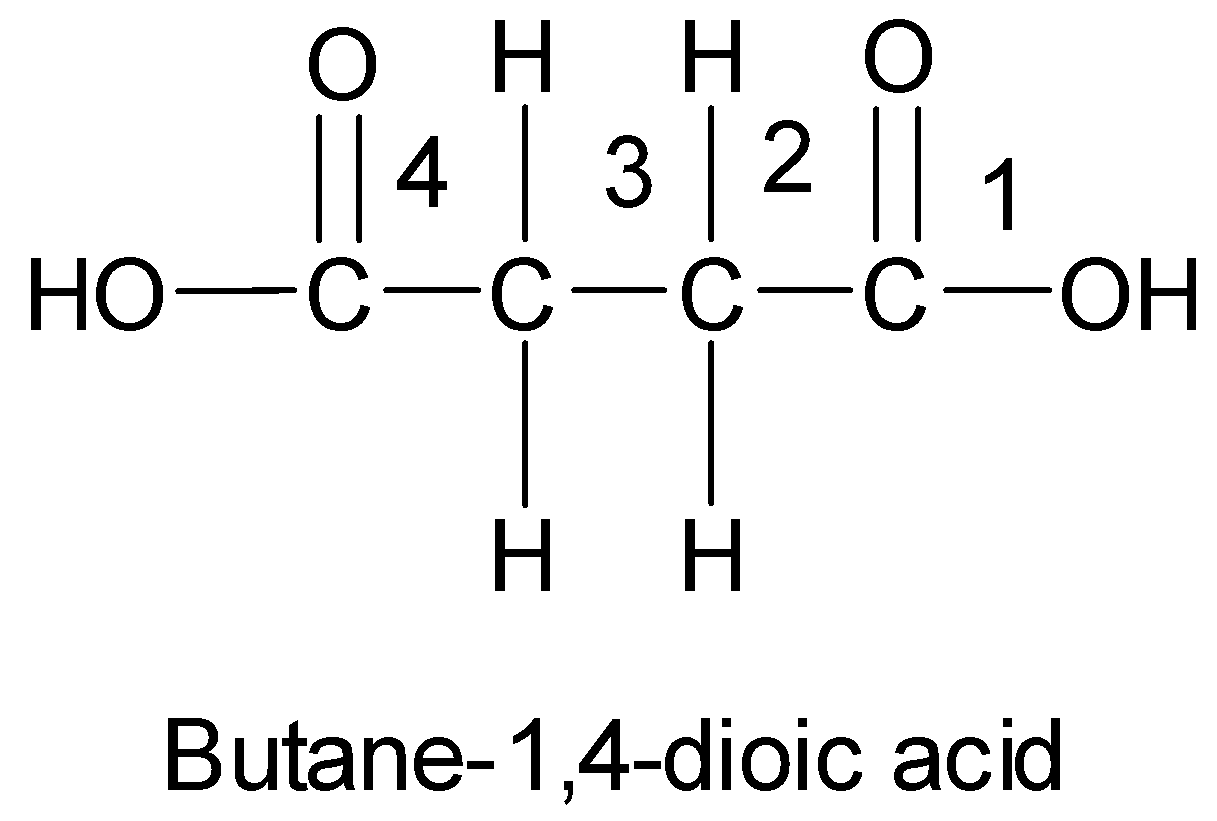
-In case of dicarboxylic acids, the IUPAC names are obtained by adding the suffix ‘dioic acid’ to the name of the parent alkane.
-The position of the carboxyl groups is indicated by Arabic numerals. For example, the following dicarboxylic acid is butane-1, 4-dioic acid.
Since the molecular formula is given to be ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}$ and there is a carboxyl ${\text{ - COOH}}$ group present, therefore, the remaining part of the compound has the ${{\text{C}}_3}{{\text{H}}_7}$ group.
Complete step by step answer:
The compound is a carboxylic acid having the formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{ - COOH}}$ .
The ${{\text{C}}_3}{{\text{H}}_7}$ group is propyl group which can also be written as ${\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}$ group.
Therefore, the structural formula of the given compound is

Or,

-According to IUPAC nomenclature, if organic compounds contain one principal functional group, then the longest continuous chain of carbon atoms containing the principal functional group is selected.
-If the functional group is such that a carbon atom is already present in it, such as the carboxyl functional group, then the numbering of the carbon chain is done in such a way that this functional group gets the lowest position of 1. While naming the compound, the position 1 is not written with such a group.
-For unsubstituted monocarboxylic acids, the IUPAC name is obtained by changing the suffix ‘ane’ of the corresponding alkane to ‘oic acid’ or in other words, it is ‘alkanoic acid’.
-Now, let us see the structure of the given acid. The carboxyl group is given the position 1 which is omitted while naming. Numbering in this way, we see that there are total 4 carbon atoms involved and so we get:

So, the IUPAC name is Butanoic acid.
Note:
-In case of dicarboxylic acids, the IUPAC names are obtained by adding the suffix ‘dioic acid’ to the name of the parent alkane.
-The position of the carboxyl groups is indicated by Arabic numerals. For example, the following dicarboxylic acid is butane-1, 4-dioic acid.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




