
Write the state of hybridization of carbon in following compounds and shapes of each of the molecules
A) $ C{H_3}Cl $
B) $ HCON{H_2} $
Answer
489.9k+ views
Hint: The IUPAC nomenclature of $ C{H_3}Cl $ is 1-chloromethane and the common name is chloromethane. It is toxic gas and is commonly used as a refrigerant and also as a local anaesthetic. $ HCON{H_2} $ has the IUPAC name as formamide and the common name is methanamide. It is used in the manufacture of formate esters and as an ionizing solvent.
Complete answer:
To find the hybridisation of any compound we will use this simple formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
Consider the first molecule given:
A) $ C{H_3}Cl $ : Using the formula to find out the hybridisation of the molecule.
$ Hybridisation = \dfrac{{4 + 3 + 1}}{2} = 4 $
Hence the hybridisation would be $ s{p^3} $ with the geometry to be tetrahedral. There are zero lone pairs in the Carbon atom of the molecule hence the shape of the molecule will also be tetrahedral itself. The structure of the molecule is as follows:
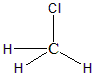
B) $ HCON{H_2} $ : This is an organic molecule, hence we can find the hybridisation using steric numbers. The formula for finding steric number is:
$ S.N = Lone{\text{ }}Pairs{\text{ }}on{\text{ }}Central{\text{ }}Atom + No.of{\text{ }}atoms{\text{ }}bonded{\text{ }}to{\text{ }}the{\text{ }}central{\text{ }}atom $
The Carbonyl carbon in Formamide has zero lone pairs, since all four valencies of carbon are occupied. The steric number for Carbon in Formamide is $ = 0 + 3 = 3 $
From the table above the hybridisation of Carbon in Formamide would be $ s{p^2} $ and the geometry would be trigonal planar. The structure of Formamide is:
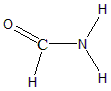
Therefore, the hybridisation of Carbon in $ C{H_3}Cl $ and $ HCON{H_2} $ is $ s{p^3} $ and $ s{p^2} $ respectively.
Note:
If there are lone pairs present on the central atom, the Hybridisation and geometry of the molecule will remain the same, their shape will change accordingly. For example, the shape of a molecule having Tetrahedral geometry with one L.P will be Pyramidal shaped. Similarly, molecules with Trigonal Planar geometry and one L.P will have angular shape.
Complete answer:
To find the hybridisation of any compound we will use this simple formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
| Answer Obtained/Steric Number | Hybridisation | Geometry |
| 2 | sp | Linear |
| 3 | $ s{p^2} $ | Trigonal Planar |
| 4 | $ s{p^3} $ | Tetrahedral |
| 5 | $ s{p^3}d $ | Trigonal Bipyramidal |
| 6 | $ s{p^3}{d^2} $ | Octahedral |
Consider the first molecule given:
A) $ C{H_3}Cl $ : Using the formula to find out the hybridisation of the molecule.
$ Hybridisation = \dfrac{{4 + 3 + 1}}{2} = 4 $
Hence the hybridisation would be $ s{p^3} $ with the geometry to be tetrahedral. There are zero lone pairs in the Carbon atom of the molecule hence the shape of the molecule will also be tetrahedral itself. The structure of the molecule is as follows:

B) $ HCON{H_2} $ : This is an organic molecule, hence we can find the hybridisation using steric numbers. The formula for finding steric number is:
$ S.N = Lone{\text{ }}Pairs{\text{ }}on{\text{ }}Central{\text{ }}Atom + No.of{\text{ }}atoms{\text{ }}bonded{\text{ }}to{\text{ }}the{\text{ }}central{\text{ }}atom $
The Carbonyl carbon in Formamide has zero lone pairs, since all four valencies of carbon are occupied. The steric number for Carbon in Formamide is $ = 0 + 3 = 3 $
From the table above the hybridisation of Carbon in Formamide would be $ s{p^2} $ and the geometry would be trigonal planar. The structure of Formamide is:

Therefore, the hybridisation of Carbon in $ C{H_3}Cl $ and $ HCON{H_2} $ is $ s{p^3} $ and $ s{p^2} $ respectively.
Note:
If there are lone pairs present on the central atom, the Hybridisation and geometry of the molecule will remain the same, their shape will change accordingly. For example, the shape of a molecule having Tetrahedral geometry with one L.P will be Pyramidal shaped. Similarly, molecules with Trigonal Planar geometry and one L.P will have angular shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




