
Write the ${{\text{S}}_{\text{N}}}{\text{2}}$ mechanism for the conversion of methyl chloride to methyl alcohol.
Answer
548.4k+ views
Hint:
${{\text{S}}_{\text{N}}}{\text{2}}$ reactions are the abbreviation of the bimolecular nucleophilic substitution reaction in which the transition state of the reaction involves two species/components.
Complete step by step solution:
In the bimolecular nucleophilic conversion of the methyl chloride to methyl alcohol, the reagent added is either aqueous sodium hydroxide or aqueous potassium hydroxide. The hydroxyl ions formed in the medium attack the methyl carbon atom from the backside of the molecule or form the rear end leading to the inversion of the chiral central for chiral compounds. As the bond between the carbon atom of methyl chloride and the oxygen atom of the hydroxyl group strengthens up, the bond between the carbon and the chlorine in the methyl group weakens and the old bond is broken and the new bond is formed at the same time. Hence methyl alcohol is formed from methyl chloride.
${\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl }}\xrightarrow{{{\text{aq}}{\text{.KOH}}}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{OH}}$
Mechanism of the reaction is as follows:
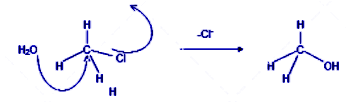
The nucleophile attacks from the backside of the molecule.
Note:
The bimolecular nucleophilic substitution reaction takes place with an inversion of the chiral centre for the chiral compounds. These reactions generally take place in polar aprotic solvents as polar protic solvents form hydrogen bonds with the nucleophile resisting it from attacking the carbon atom. Steric factors are very important for these reactions and the rate of the reaction is as follows: Primary group> Secondary Group> Tertiary group.
The transition state in which the bond breaking and bond making takes place is a penta coordinated state in which the hybridization of the carbon atom is approximately ${\text{s}}{{\text{p}}^{\text{2}}}$. Polar aprotic solvents like tetrahydrofuran, DMSO, acetone, DMF, etc are better for these reactions than polar protic solvents.
${{\text{S}}_{\text{N}}}{\text{2}}$ reactions are the abbreviation of the bimolecular nucleophilic substitution reaction in which the transition state of the reaction involves two species/components.
Complete step by step solution:
In the bimolecular nucleophilic conversion of the methyl chloride to methyl alcohol, the reagent added is either aqueous sodium hydroxide or aqueous potassium hydroxide. The hydroxyl ions formed in the medium attack the methyl carbon atom from the backside of the molecule or form the rear end leading to the inversion of the chiral central for chiral compounds. As the bond between the carbon atom of methyl chloride and the oxygen atom of the hydroxyl group strengthens up, the bond between the carbon and the chlorine in the methyl group weakens and the old bond is broken and the new bond is formed at the same time. Hence methyl alcohol is formed from methyl chloride.
${\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl }}\xrightarrow{{{\text{aq}}{\text{.KOH}}}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{OH}}$
Mechanism of the reaction is as follows:

The nucleophile attacks from the backside of the molecule.
Note:
The bimolecular nucleophilic substitution reaction takes place with an inversion of the chiral centre for the chiral compounds. These reactions generally take place in polar aprotic solvents as polar protic solvents form hydrogen bonds with the nucleophile resisting it from attacking the carbon atom. Steric factors are very important for these reactions and the rate of the reaction is as follows: Primary group> Secondary Group> Tertiary group.
The transition state in which the bond breaking and bond making takes place is a penta coordinated state in which the hybridization of the carbon atom is approximately ${\text{s}}{{\text{p}}^{\text{2}}}$. Polar aprotic solvents like tetrahydrofuran, DMSO, acetone, DMF, etc are better for these reactions than polar protic solvents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




