
Write the resonance structures of $ C{H_3}CO{O^ - } $ and show the movement of electrons by curved arrows.
Answer
487.5k+ views
Hint: In resonance structure, the electrons move from one atom to another, i.e. resonate. These are possible Lewis structures of the same molecule, it is believed that the molecule will exist in any of these structures at a given point in time. The arrows represent the movement of electrons.
Complete answer:
Two resonance structures of $ C{H_3}CO{O^ - } $ are possible. The negative charge and the electron move between the two oxygen atoms present in the structure.
For drawing the resonance structures, we need to first draw the structure and then put the unshared pair of valence electrons on their designated atoms and then draw the arrow to represent the electron movement.
The resonance structures of $ C{H_3}CO{O^ - } $ are -
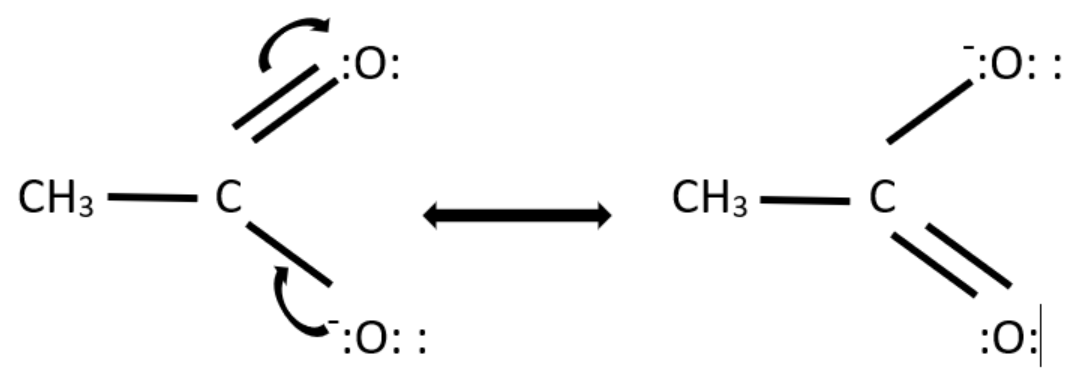
Hence, the given diagram represents the resonance structure of $ C{H_3}CO{O^ - } $ and the arrows represent the movement of electrons.
Note:
The resonance in chemistry is also called mesomerism, the concept of describing the bondings in molecules and predicting the possible structures of that particular molecule. The resonance structures or mesomeric forms are very effective when the structure of a molecule cannot be described satisfactorily using a single Lewis dot structure.
Complete answer:
Two resonance structures of $ C{H_3}CO{O^ - } $ are possible. The negative charge and the electron move between the two oxygen atoms present in the structure.
For drawing the resonance structures, we need to first draw the structure and then put the unshared pair of valence electrons on their designated atoms and then draw the arrow to represent the electron movement.
The resonance structures of $ C{H_3}CO{O^ - } $ are -

Hence, the given diagram represents the resonance structure of $ C{H_3}CO{O^ - } $ and the arrows represent the movement of electrons.
Note:
The resonance in chemistry is also called mesomerism, the concept of describing the bondings in molecules and predicting the possible structures of that particular molecule. The resonance structures or mesomeric forms are very effective when the structure of a molecule cannot be described satisfactorily using a single Lewis dot structure.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




