
Write the reaction involved in the extraction of copper from its ore by electrolytic refining.
Answer
578.1k+ views
Hint: Electrolytic refining is the final step of purification in the whole process of obtaining copper metal from its ore. In this process purification is done using copper sulfate solution and deposition of pure copper takes place at cathode and overall a redox reaction takes place.
Complete step by step answer:
In an electrolytic cell, anode is made of impure copper and cathode is made of pure copper and they are dipped in the solution of copper sulphate.
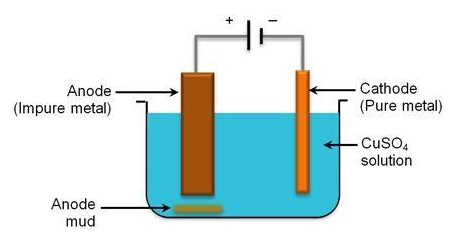
When current passed through the solution, copper ions from solution moved towards cathode and sulphate ions move towards anode.
Copper ions get reduced and get deposited on cathode.
At cathode: $C{{u}^{2+}}(aq)+2{{e}^{-}}\to Cu(s)$
Sulfate ions make sure that the copper atoms present in anode rod get oxidized and come into the solution.
At canode: $Cu(s)\to Cu(aq)+2{{e}^{-}}$
So overall copper ions from the solution are getting deposited on the cathode and copper atoms from the anode are coming into the solution. Therefore we are getting pure copper at cathode. With time, the cathode rod will get thicker because of deposition and the anode rod gets thinner because of dissolution.
Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) does not go into solution as ions. It stays as a metal and falls to the bottom of the cell as "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold.
Additional information:
Chalcopyrite, chalcocite, covellite, bornite are the major sulfide ores of copper from which copper is usually obtained.
Note: when copper is obtained from its ore chalcopyrite, first of all froth flotation is done to increase the concentration of ore. After this, copper is heated in bassemerisar along with silica in presence of air. From this we get blister copper that we take for electrolytic refining.
Complete step by step answer:
In an electrolytic cell, anode is made of impure copper and cathode is made of pure copper and they are dipped in the solution of copper sulphate.

When current passed through the solution, copper ions from solution moved towards cathode and sulphate ions move towards anode.
Copper ions get reduced and get deposited on cathode.
At cathode: $C{{u}^{2+}}(aq)+2{{e}^{-}}\to Cu(s)$
Sulfate ions make sure that the copper atoms present in anode rod get oxidized and come into the solution.
At canode: $Cu(s)\to Cu(aq)+2{{e}^{-}}$
So overall copper ions from the solution are getting deposited on the cathode and copper atoms from the anode are coming into the solution. Therefore we are getting pure copper at cathode. With time, the cathode rod will get thicker because of deposition and the anode rod gets thinner because of dissolution.
Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) does not go into solution as ions. It stays as a metal and falls to the bottom of the cell as "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold.
Additional information:
Chalcopyrite, chalcocite, covellite, bornite are the major sulfide ores of copper from which copper is usually obtained.
Note: when copper is obtained from its ore chalcopyrite, first of all froth flotation is done to increase the concentration of ore. After this, copper is heated in bassemerisar along with silica in presence of air. From this we get blister copper that we take for electrolytic refining.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




