
Write the product for the given conversion:
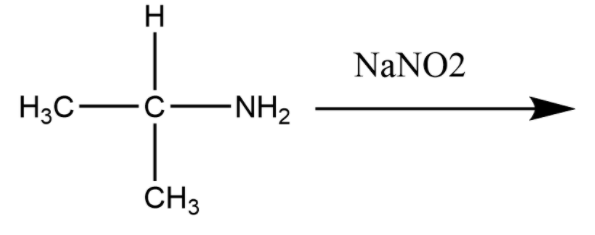

Answer
556.2k+ views
Hint: The substrate given above is a primary amine, it is having two hydrogens and one carbon atom connected with it. Now, when this primary amine will react with sodium nitrite $NaN{O_2}$ it will form a diazonium salt the amine group will convert to make diazonium salt. Now there is a concept that when we are talking about primary amine which are non aromatic in nature they complete the reaction and give hydrolysis products.
Complete step-by-step answer:
We have a primary amine as substrate which will undergo an organic reaction and give diazonium salt, it looks like that there are two nitrogen atoms connected with a double bond and a chlorine atom with them. This reagent sodium nitrite $NaN{O_2}$ works with hydrochloric acid $HCl$ and forms a diazonium salt.
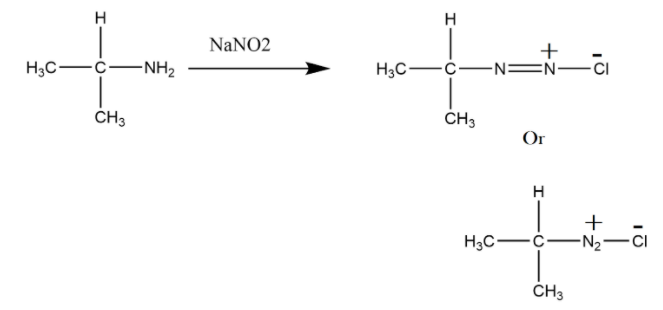
The diazonium salt can be made as shown above or we can write nitrogen together. In the next step this diazonium salt will convert into alcohol by hydrolysis, if water is not given the product will be formed by absorbing moisture. This is because the salt formed is unstable when it forms non-aromatically.
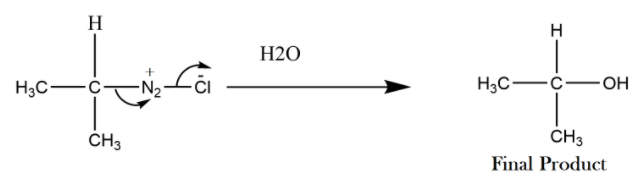
As we see that the product formed above is alcohol, so primary amine gets changed to primary alcohol when it reacts with sodium nitrite.
Note: If in place of non aromatic primary amine, we have given an aromatic amine like for example take aniline so if the same reagent reacts with aniline the reaction get stop after forming the diazonium salt, it didn’t proceed the reaction further till hydrolysis product. This is the main exception for reagent $NaN{O_2}$ or we can also call it a diazotization reaction.
Complete step-by-step answer:
We have a primary amine as substrate which will undergo an organic reaction and give diazonium salt, it looks like that there are two nitrogen atoms connected with a double bond and a chlorine atom with them. This reagent sodium nitrite $NaN{O_2}$ works with hydrochloric acid $HCl$ and forms a diazonium salt.

The diazonium salt can be made as shown above or we can write nitrogen together. In the next step this diazonium salt will convert into alcohol by hydrolysis, if water is not given the product will be formed by absorbing moisture. This is because the salt formed is unstable when it forms non-aromatically.

As we see that the product formed above is alcohol, so primary amine gets changed to primary alcohol when it reacts with sodium nitrite.
Note: If in place of non aromatic primary amine, we have given an aromatic amine like for example take aniline so if the same reagent reacts with aniline the reaction get stop after forming the diazonium salt, it didn’t proceed the reaction further till hydrolysis product. This is the main exception for reagent $NaN{O_2}$ or we can also call it a diazotization reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




