
How do you write the orbital diagram for sulphur?
Answer
564.6k+ views
Hint: Orbital diagrams are pictorial illustrations of the electrons present within an atom. The formation of orbital diagrams follow the AufBau Principle, which shows each electron occupying the lowest energy orbital.
Complete answer:
- To obtain the orbital diagram for sulphur, we must first understand the basic rules of AufBau Principle.
- The AufBau Principle clearly states that the electrons tend to fill lower-energy atomic orbitals before filling higher-energy orbitals.
- In German, AufBau itself means to “build-up”. And so, this rule is applicable to predict the electronic configuration for atoms or ions.
- In order to obtain the electronic configuration of sulphur, we first need to mention the total number of electrons that are present in the sulphur atom, which is 16 electrons.
- In the electronic configuration of sulphur, the first two electrons will occupy 1s orbital. Since 1s orbital can hold only two electrons, the following electrons will go to 2p orbital.
- Now, 2p orbital is able to hold up to 6 electrons. So, 6 electrons will rest into 2p orbital and the following electrons will be put in 3s orbitals.
- After 3s orbital is full, we’ll move the remaining 4 electrons to 3p orbital.
- Therefore, the electronic configuration for sulphur will be
$1s^{2}$ $2s^{2}$ $2p^{6}$ $3s^{2}$ $3p^{4}$
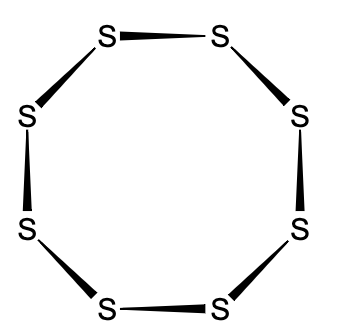
- Using this configuration, a sulphur atom forms a molecule with 8 sulphur atoms.
Note:
The electronic configuration is an easy way to understand how electrons are arranged around the nucleus of an atom. This makes it easier to comprehend and predict the interaction between atoms to form chemical bonds.
Complete answer:
- To obtain the orbital diagram for sulphur, we must first understand the basic rules of AufBau Principle.
- The AufBau Principle clearly states that the electrons tend to fill lower-energy atomic orbitals before filling higher-energy orbitals.
- In German, AufBau itself means to “build-up”. And so, this rule is applicable to predict the electronic configuration for atoms or ions.
- In order to obtain the electronic configuration of sulphur, we first need to mention the total number of electrons that are present in the sulphur atom, which is 16 electrons.
- In the electronic configuration of sulphur, the first two electrons will occupy 1s orbital. Since 1s orbital can hold only two electrons, the following electrons will go to 2p orbital.
- Now, 2p orbital is able to hold up to 6 electrons. So, 6 electrons will rest into 2p orbital and the following electrons will be put in 3s orbitals.
- After 3s orbital is full, we’ll move the remaining 4 electrons to 3p orbital.
- Therefore, the electronic configuration for sulphur will be
$1s^{2}$ $2s^{2}$ $2p^{6}$ $3s^{2}$ $3p^{4}$
- Using this configuration, a sulphur atom forms a molecule with 8 sulphur atoms.

Note:
The electronic configuration is an easy way to understand how electrons are arranged around the nucleus of an atom. This makes it easier to comprehend and predict the interaction between atoms to form chemical bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




