
Write the names of the monomers used for getting the following polymers.
A) ${\text{Nylon 6,6}}$
B) Glyptal
C) Bakelite
D) Terylene
Answer
582.6k+ views
Hint: A copolymer is made from two (or more) types of monomer units. In the repeating structural unit of a copolymer, two (or more) types of monomer unit are present.
The polymers (i), ${\text{Nylon 6,6}}$ Glyptal, bakelite and Terylene are copolymers.
Complete answer:
A) ${\text{Nylon 6,6}}$ is a polyamide made from condensation polymerization of di carboxylic acid with diamine. Here, 6,6 represents that both monomers contain six carbon atoms each.
Glyptal is a polyester made from condensation polymerization of di carboxylic acid with diol.
Bakelite is a condensation polymer of phenol with an aldehyde.
Terylene is a polyester made from condensation polymerization of diester with diol.
Write the names of the monomers for each polymer as shown below:
${\text{Nylon 6,6}}$is a polyamide made from condensation polymerization of adipic acid (a dicarboxylic acid) with hexamethylenediamine (a diamine).
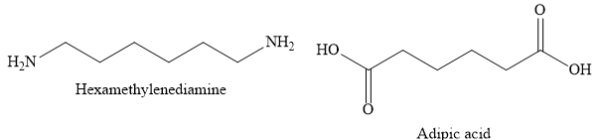
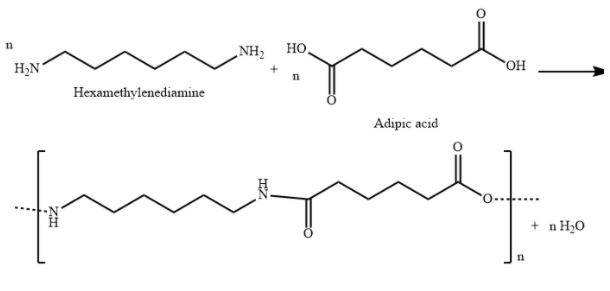
${\text{Nylon 6,6}}$ is used in airbags, tires, ropes, parachutes, swimwear etc.
Glyptal is a polyester made from condensation polymerization of ethylene glycol (a diol) and phthalic acid (a dicarboxylic acid).

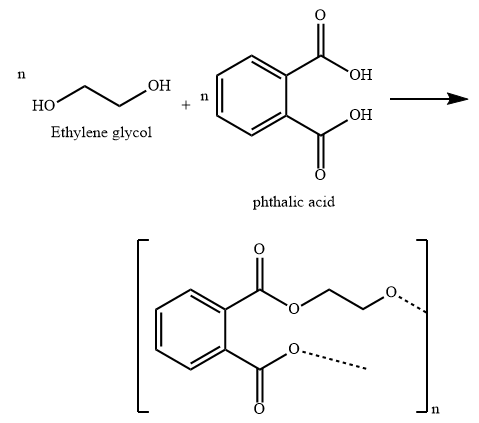
B) Glyptal is the polymer used in the manufacture of paints and lacquers. It is also used as a binding material and in preparation of mixed plastics. It is a copolymer of ethylene glycol and phthalic acid. It is obtained by condensation polymerization and is a polyester.
In the formation of Glyptal, H atom of hydroxyl group of ethylene glycol combines with hydroxyl group of carboxylic function group of phthalic acid and a molecule of water is eliminated.
C) Bakelite polymer is made from monomers Phenol and formaldehyde.
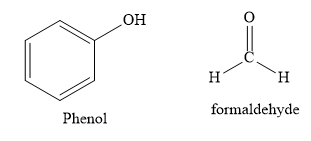
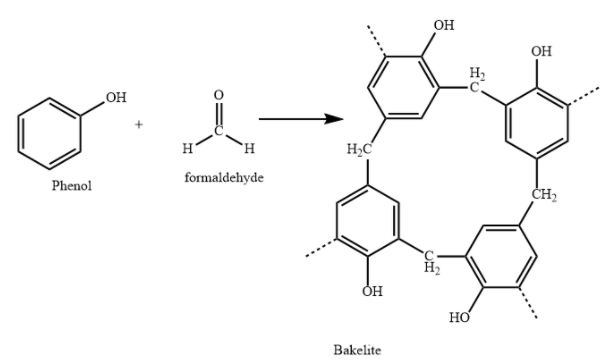
Bakelite is used in making combs, phonograph records, handles of various utensils and electrical switches. It is a copolymer of phenol and formaldehyde. It is prepared by condensation polymerization.
D) Terylene is a polyester made from condensation polymerisation of ethylene glycol (diol) and dimethyl terephthalate (diester).

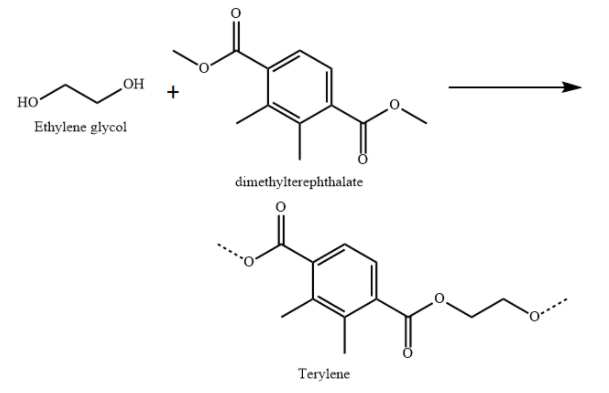
Terylene is used in plastic bottles, fabrics and clothes.
Note: Do not mix nylon 6,6 with nylon 6. is a polyamide made from condensation polymerization of adipic acid with hexamethylenediamine.
The polymers (i), ${\text{Nylon 6,6}}$ Glyptal, bakelite and Terylene are copolymers.
Complete answer:
A) ${\text{Nylon 6,6}}$ is a polyamide made from condensation polymerization of di carboxylic acid with diamine. Here, 6,6 represents that both monomers contain six carbon atoms each.
Glyptal is a polyester made from condensation polymerization of di carboxylic acid with diol.
Bakelite is a condensation polymer of phenol with an aldehyde.
Terylene is a polyester made from condensation polymerization of diester with diol.
Write the names of the monomers for each polymer as shown below:
${\text{Nylon 6,6}}$is a polyamide made from condensation polymerization of adipic acid (a dicarboxylic acid) with hexamethylenediamine (a diamine).


${\text{Nylon 6,6}}$ is used in airbags, tires, ropes, parachutes, swimwear etc.
Glyptal is a polyester made from condensation polymerization of ethylene glycol (a diol) and phthalic acid (a dicarboxylic acid).


B) Glyptal is the polymer used in the manufacture of paints and lacquers. It is also used as a binding material and in preparation of mixed plastics. It is a copolymer of ethylene glycol and phthalic acid. It is obtained by condensation polymerization and is a polyester.
In the formation of Glyptal, H atom of hydroxyl group of ethylene glycol combines with hydroxyl group of carboxylic function group of phthalic acid and a molecule of water is eliminated.
C) Bakelite polymer is made from monomers Phenol and formaldehyde.


Bakelite is used in making combs, phonograph records, handles of various utensils and electrical switches. It is a copolymer of phenol and formaldehyde. It is prepared by condensation polymerization.
D) Terylene is a polyester made from condensation polymerisation of ethylene glycol (diol) and dimethyl terephthalate (diester).


Terylene is used in plastic bottles, fabrics and clothes.
Note: Do not mix nylon 6,6 with nylon 6. is a polyamide made from condensation polymerization of adipic acid with hexamethylenediamine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




