
Write the name of following compounds:
(A) ${C_6}{H_5}{N_2}{H^ + }SO_4^ - $
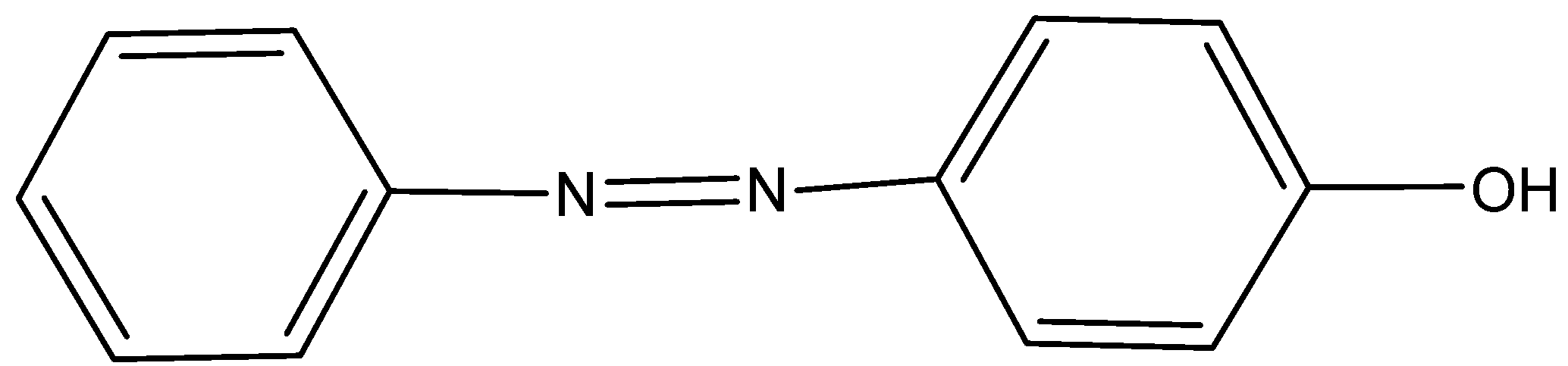
(B) Ref. image

Answer
510.3k+ views
Hint: We know that Diazonium particles are available in arrangements, for example, benzenediazonium chloride arrangement. They contain a \[{N_2}^ + \] group. On account of benzenediazonium chloride, this is joined to a benzene ring.
Complete answer:
We must need to remember that the name of the given compound A ${C_6}{H_5}{N_2}{H^ + }SO_4^ - $ is Benzenediazonium hydrogen sulfate is like Benzenediazonium hydrogen chloride \[Ph - {N_2} - Cl\]. The name of the given compound B is (4-phenyldiazenyl) phenol is obtained by azo coupling.
Additional information:
Now we can discuss about the preparation of diazonium salts:
Watery arrangements of diazonium chloride salts, generally set up from the aniline, sodium nitrite, and hydrochloric corrosive, are precarious at room temperature and are traditionally set up at \[0-5^\circ C\]. It is regularly favored that the diazonium salt stays in arrangement, yet they do tend to supersaturate. Administrators have been harmed or even executed by a sudden crystallization of the salt followed by its detonation. Because of these risks, diazonium compounds are generally not separated. Rather they are utilized in situ
Note:
Let’s we see about the significance of Diazonium Salts:
They discover application in the color and shade enterprises and are utilized to deliver colored textures.
Because of their property of separating close to the bright light, they are utilized in record propagation
They are valuable in the blend of a huge assortment of natural mixtures, particularly aryl subsidiaries.
Direct halogenation is definitely not a reasonable technique for planning aryl iodides and fluorides. Nucleophilic replacement of chlorine in chlorobenzene by a cyano bunch is absurd. Be that as it may, diazonium salts can without much of a stretch be utilized to deliver cyanobenzene.
It is preposterous to expect to plan subbed sweet-smelling compounds by direct replacement in benzene. For these mixtures, we utilize the substitution of the diazo gathering in diazonium salts.
Complete answer:
We must need to remember that the name of the given compound A ${C_6}{H_5}{N_2}{H^ + }SO_4^ - $ is Benzenediazonium hydrogen sulfate is like Benzenediazonium hydrogen chloride \[Ph - {N_2} - Cl\]. The name of the given compound B is (4-phenyldiazenyl) phenol is obtained by azo coupling.
Additional information:
Now we can discuss about the preparation of diazonium salts:
Watery arrangements of diazonium chloride salts, generally set up from the aniline, sodium nitrite, and hydrochloric corrosive, are precarious at room temperature and are traditionally set up at \[0-5^\circ C\]. It is regularly favored that the diazonium salt stays in arrangement, yet they do tend to supersaturate. Administrators have been harmed or even executed by a sudden crystallization of the salt followed by its detonation. Because of these risks, diazonium compounds are generally not separated. Rather they are utilized in situ
Note:
Let’s we see about the significance of Diazonium Salts:
They discover application in the color and shade enterprises and are utilized to deliver colored textures.
Because of their property of separating close to the bright light, they are utilized in record propagation
They are valuable in the blend of a huge assortment of natural mixtures, particularly aryl subsidiaries.
Direct halogenation is definitely not a reasonable technique for planning aryl iodides and fluorides. Nucleophilic replacement of chlorine in chlorobenzene by a cyano bunch is absurd. Be that as it may, diazonium salts can without much of a stretch be utilized to deliver cyanobenzene.
It is preposterous to expect to plan subbed sweet-smelling compounds by direct replacement in benzene. For these mixtures, we utilize the substitution of the diazo gathering in diazonium salts.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




