
Write the molecular formula of benzene and state the number of double bonds in the structure.
Answer
527.1k+ views
Hint: Benzene is a colourless organic compound having sweet chromatic odour it is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. As it contains only carbon are hydrogen atoms, benzene is classed as a hydrocarbon it is highly flammable. Benzene is a toxic chemical so it is carcinogenic i.e. causes cancer.
Complete step by step answer:
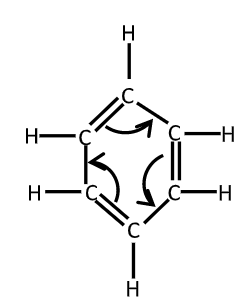
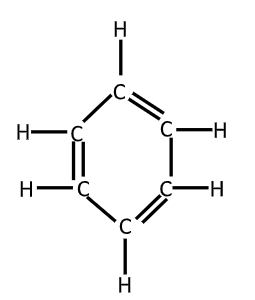
As the benzene is composed of $6$ carbon – atoms and $6 - $hydrogen atom joined in the trigonal planar ring, its molecular formula is${C_6}{H_6}$. The empirical formula for benzene was long known but it’s highly poly – unsaturated with just one hydrogen atom for exact carbon atom was challenging to determine. A.S. Coupor in $1858$ and Joseph Loschmidt in $1861$ suggested the possible structure that contained multiple double bonds or multiple rings but $100$ little evidence was then available to help chemists decide on any particular structure.
In $1865$, the German chemist Friedrich August Kekule suggested that the structure contained a ring of six carbon atoms with alternating single and double bonds.
X – ray diffraction shows that all $6$ Carbon – carbon bonds in benzene are of same lengths at $140$ pm. The C – C bond lengths are greater than double bonds $\left( {135PM} \right)$ but shorter than a single bond $\left( {147pm} \right)$ the intermediate distance is consistent with electron delocalization. The electrons for C – C bonding are distributed equally between each of the six carbon atoms. Benzene has $6$ H – atoms fever than the corresponding parent alkane hexane. The molecule is planar. The molecular orbital description involves the formation of three delocalized $\pi $ orbitals spanning all six carbon atoms white the valence bond description involves a superposition of resonance structures called aromaticity.
Note:
Critics pointed out a problem with Kekule’s original structure$\left( {1865} \right)$for benzene. Whenever benzene goes substitutions at the ortho position two distinguished isomers are resulted depending on whether the double bond or single bond existed between the carbon atoms to which the substituent were attached, however no such isomers were observed in $1872$ Kekule suggested that benzene acid two complementary structures I that these forms rapidly interconverted, so that if there were a double bond between any pair of carbon atoms at one instant, that double bond would become a single bond at the next instant and vice versa.
Complete step by step answer:
As the benzene is composed of $6$ carbon – atoms and $6 - $hydrogen atom joined in the trigonal planar ring, its molecular formula is${C_6}{H_6}$. The empirical formula for benzene was long known but it’s highly poly – unsaturated with just one hydrogen atom for exact carbon atom was challenging to determine. A.S. Coupor in $1858$ and Joseph Loschmidt in $1861$ suggested the possible structure that contained multiple double bonds or multiple rings but $100$ little evidence was then available to help chemists decide on any particular structure.
In $1865$, the German chemist Friedrich August Kekule suggested that the structure contained a ring of six carbon atoms with alternating single and double bonds.
X – ray diffraction shows that all $6$ Carbon – carbon bonds in benzene are of same lengths at $140$ pm. The C – C bond lengths are greater than double bonds $\left( {135PM} \right)$ but shorter than a single bond $\left( {147pm} \right)$ the intermediate distance is consistent with electron delocalization. The electrons for C – C bonding are distributed equally between each of the six carbon atoms. Benzene has $6$ H – atoms fever than the corresponding parent alkane hexane. The molecule is planar. The molecular orbital description involves the formation of three delocalized $\pi $ orbitals spanning all six carbon atoms white the valence bond description involves a superposition of resonance structures called aromaticity.


Note:
Critics pointed out a problem with Kekule’s original structure$\left( {1865} \right)$for benzene. Whenever benzene goes substitutions at the ortho position two distinguished isomers are resulted depending on whether the double bond or single bond existed between the carbon atoms to which the substituent were attached, however no such isomers were observed in $1872$ Kekule suggested that benzene acid two complementary structures I that these forms rapidly interconverted, so that if there were a double bond between any pair of carbon atoms at one instant, that double bond would become a single bond at the next instant and vice versa.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




