
Write the molecular and structural formulae of Thiosulphuric acid.
Answer
577.8k+ views
Hint: In oxyacid of sulfur, sulfur is the central atom exhibiting a tetrahedral structure when coordinated with oxygen. This contains at least one \[S = O\] bond and one \[S - OH\] bond. To solve this question, you must follow these steps: first, draw the molecular structure of thiosulphuric acid.
Complete step by step answer:
The molecular formula is a way to write a substance using the chemical symbol and number subscripts to mention the number of the atoms in that chemical compound. On the other hand, a condensed formula is basically like the structural formula but here it is showing that all groups are attached with one single atom.
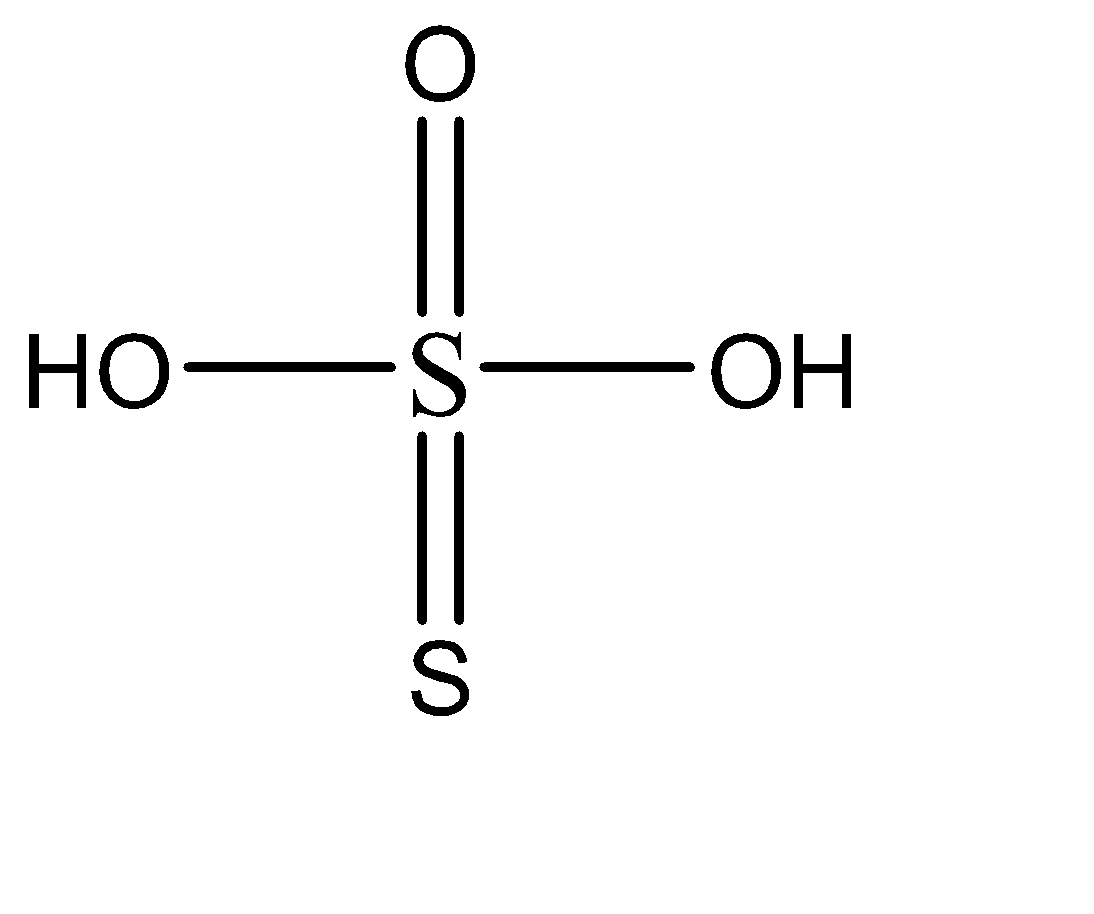
The molecular formula of thiosulfuric acid is, \[{H_2}{S_2}{O_3}\].
The molecular structure of thiosulphuric acid:
Additional information:
Now, let discuss some statements about thiosulphuric acid:
1.Each sulphur atom is in an identical oxidation state:
The central sulphur atom has formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atom has formed only one sigma and one pi bond. Hence the oxidation states of both the sulphur atoms are different.
2.There is an S = S linkage present:
As we can see in the molecular structure, there is indeed a double bond present between the two sulphur atoms.
3.One atom is in +2 and the other sulphur atom is in +4 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\]. Now, from point 2, we can say that the central sulphur shares with all the 6 electrons present in the valence shell and achieves a +6-oxidation state. While the other sulphur atom accepts the 2 electrons shared by the central sulphur atom and achieves an oxidation state of (-2).
4.There is only one replaceable hydrogen atom:
There are 2 \[ - OH\] groups present in this compound. Each \[ - OH\] group has one replaceable hydrogen. Hence the compound has 2 replaceable hydrogens.
Note: Sodium thiosulphate is an inorganic sodium salt of thiosulphuric acid, composed of sodium and thiosulphate ions in a $2:1$ ratio. It has a role as an antidote to cyanide poisoning, a nephroprotective agent, and an antifungal drug. It is used in swimming pools as a reagent in the testing of pool water, primarily to neutralize the whitening effect of chlorine present in a water sample.
Complete step by step answer:
The molecular formula is a way to write a substance using the chemical symbol and number subscripts to mention the number of the atoms in that chemical compound. On the other hand, a condensed formula is basically like the structural formula but here it is showing that all groups are attached with one single atom.
The molecular formula of thiosulfuric acid is, \[{H_2}{S_2}{O_3}\].
The molecular structure of thiosulphuric acid:

Additional information:
Now, let discuss some statements about thiosulphuric acid:
1.Each sulphur atom is in an identical oxidation state:
The central sulphur atom has formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atom has formed only one sigma and one pi bond. Hence the oxidation states of both the sulphur atoms are different.
2.There is an S = S linkage present:
As we can see in the molecular structure, there is indeed a double bond present between the two sulphur atoms.
3.One atom is in +2 and the other sulphur atom is in +4 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\]. Now, from point 2, we can say that the central sulphur shares with all the 6 electrons present in the valence shell and achieves a +6-oxidation state. While the other sulphur atom accepts the 2 electrons shared by the central sulphur atom and achieves an oxidation state of (-2).
4.There is only one replaceable hydrogen atom:
There are 2 \[ - OH\] groups present in this compound. Each \[ - OH\] group has one replaceable hydrogen. Hence the compound has 2 replaceable hydrogens.
Note: Sodium thiosulphate is an inorganic sodium salt of thiosulphuric acid, composed of sodium and thiosulphate ions in a $2:1$ ratio. It has a role as an antidote to cyanide poisoning, a nephroprotective agent, and an antifungal drug. It is used in swimming pools as a reagent in the testing of pool water, primarily to neutralize the whitening effect of chlorine present in a water sample.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




