
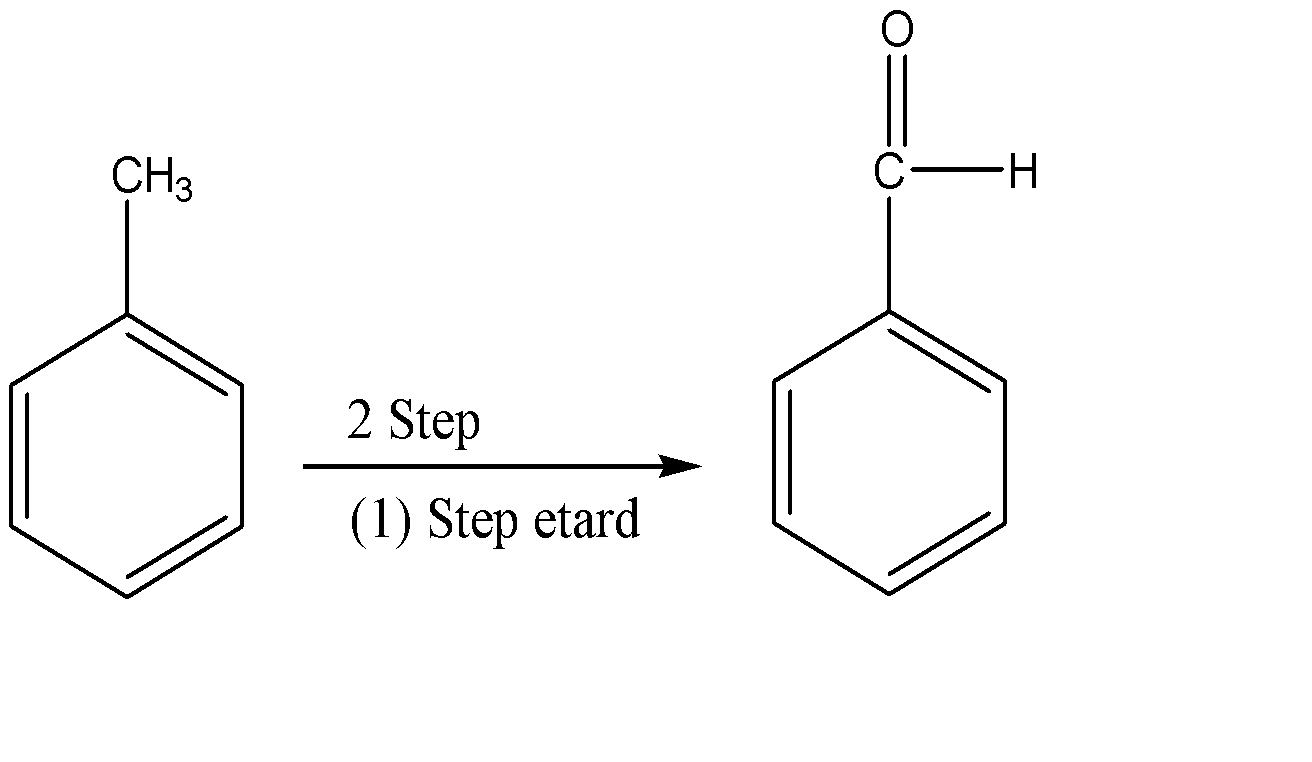
Write the mechanism for Étard Process

Answer
577.8k+ views
Hint: Étard reaction is one of the commonly used processes that is used for oxidizing aromatic methyl groups. This reaction involves an aromatic methyl group as a reactant which is oxidized by using chromyl chloride as a catalyst.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The first step in the Étard reaction begins with the reaction of chromyl chloride with an allyl – alkene hydrogen reaction. This results in the formation of a precipitate called the Étard Complex. A carboxylic acid is then obtained by decomposing this precipitate in a reducing environment. Sodium sulphite is generally used for providing this reducing environment. Reducing the environment is necessary because we do not want the precipitate to undergo oxidation. This results in the formation of the desired aldehydic product.
The mechanism of this reaction can be discussed as follows:
First of all, we use chromyl chloride to form a Étard complex, which is basically an alkene – alkyl hydrogen reaction. It can be represented as follows:
After the purification of this Étard complex is done, it undergoes decomposition. The purification process involves a pericyclic reaction where via an uncatalyzed intermolecular process, we try to obtain a product which has one sigma bond changed to another sigma bond. The mechanism for this entire part can be represented as:
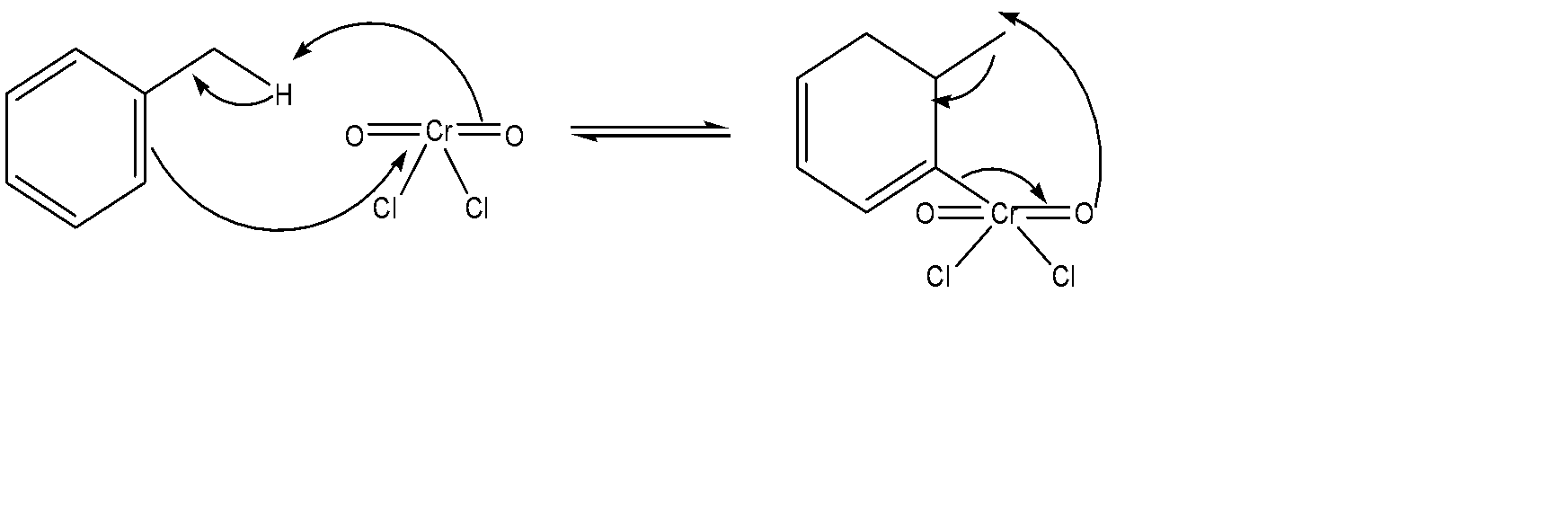
Hence, this is the mechanism for the Étard Process
Note: The Étard complex is not allowed to oxidize because that would result in the formation of a carboxylic acid. We use saturated forms of sodium sulphite to create a reducing environment, and one of the most common solvents used in this process is carbon tetrachloride.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The first step in the Étard reaction begins with the reaction of chromyl chloride with an allyl – alkene hydrogen reaction. This results in the formation of a precipitate called the Étard Complex. A carboxylic acid is then obtained by decomposing this precipitate in a reducing environment. Sodium sulphite is generally used for providing this reducing environment. Reducing the environment is necessary because we do not want the precipitate to undergo oxidation. This results in the formation of the desired aldehydic product.
The mechanism of this reaction can be discussed as follows:
First of all, we use chromyl chloride to form a Étard complex, which is basically an alkene – alkyl hydrogen reaction. It can be represented as follows:
After the purification of this Étard complex is done, it undergoes decomposition. The purification process involves a pericyclic reaction where via an uncatalyzed intermolecular process, we try to obtain a product which has one sigma bond changed to another sigma bond. The mechanism for this entire part can be represented as:

Hence, this is the mechanism for the Étard Process
Note: The Étard complex is not allowed to oxidize because that would result in the formation of a carboxylic acid. We use saturated forms of sodium sulphite to create a reducing environment, and one of the most common solvents used in this process is carbon tetrachloride.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




