
Write the major product of the following reaction:
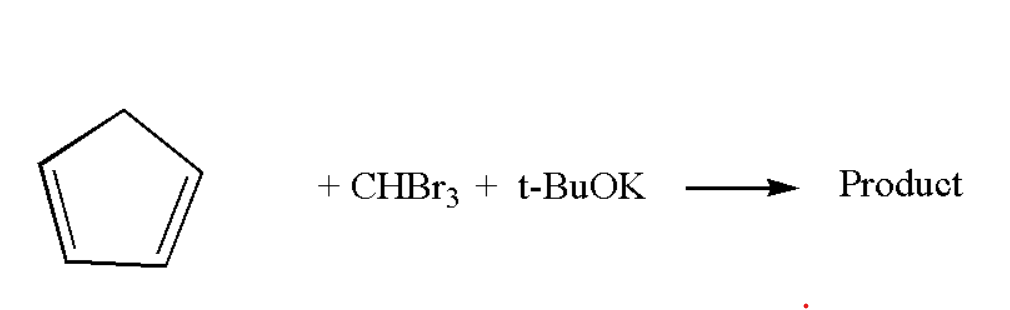
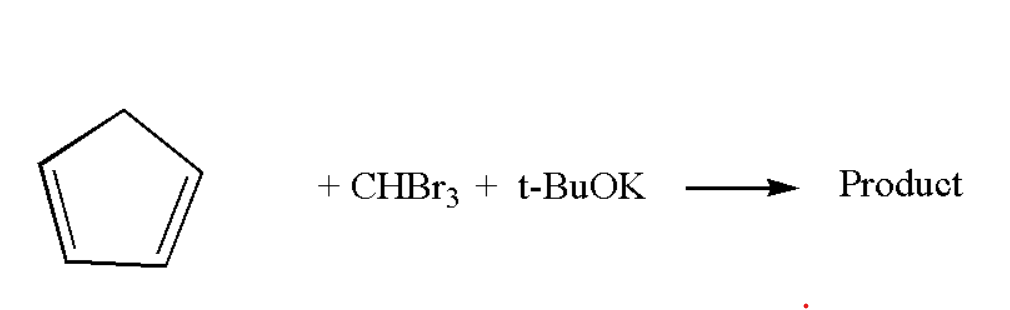
Answer
498k+ views
Hint: $ {\text{t - BuOK }} $ is a dehydrating agent. It is known as tertiary butoxide. When tertiary butyl reacts with $ {\text{KOH}} $ , then $ {\text{t - BuOK }} $ is formed. Also $ {\text{CHB}}{{\text{r}}_3} $ is known as bromoform. It gives us free radical $ :CB{r_2} $ which further reacts with the carbon ring.
Complete Step By Step Answer:
When the bromoform reacts with the $ {\text{t - BuOK }} $ , it gives us the $ :CB{r_2} $ . Now $ :CB{r_2} $ will react with the given carbon ring. The reaction will proceed in the following steps as:
$ 1. $ $ :CB{r_2} $ thus formed will approach the carbon ring as,
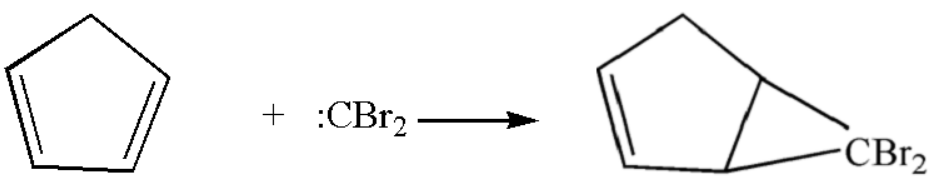
$ 2. $ Now the ring chlorine atoms will leave the compound leaving behind the methyl group which will be attached to the ring by ring expansion.
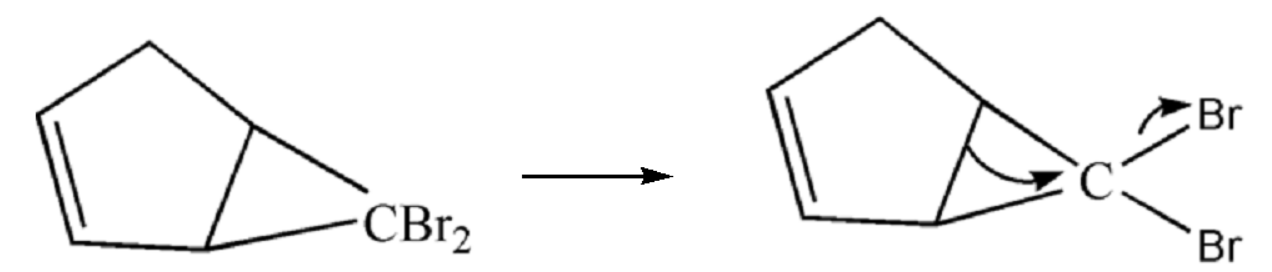
$ 3. $ Now the cyclic compound will undergo ring expansion and hence it will form a six member ring. Bromine will leave the site and the methyl group gets bonded with the carbon of the ring.
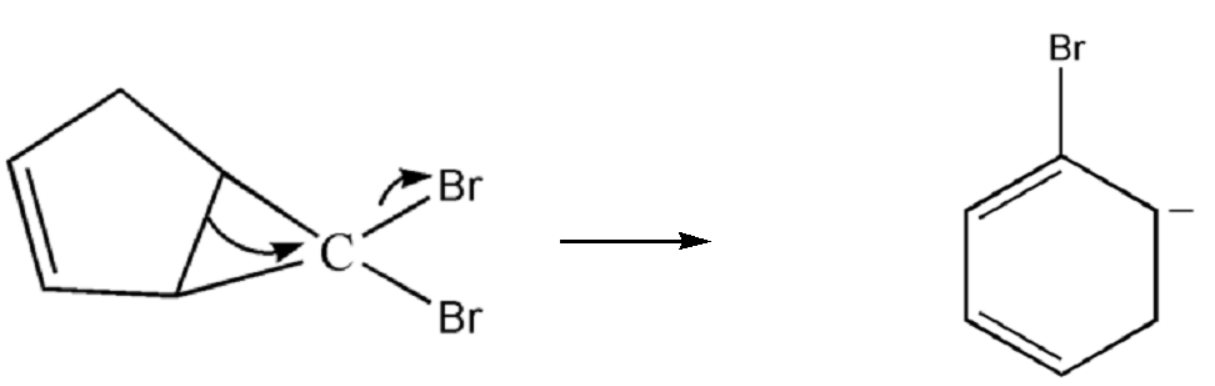
$ 4. $ Now the stabilisation of charge takes place which in turn gives us a ring of benzene.
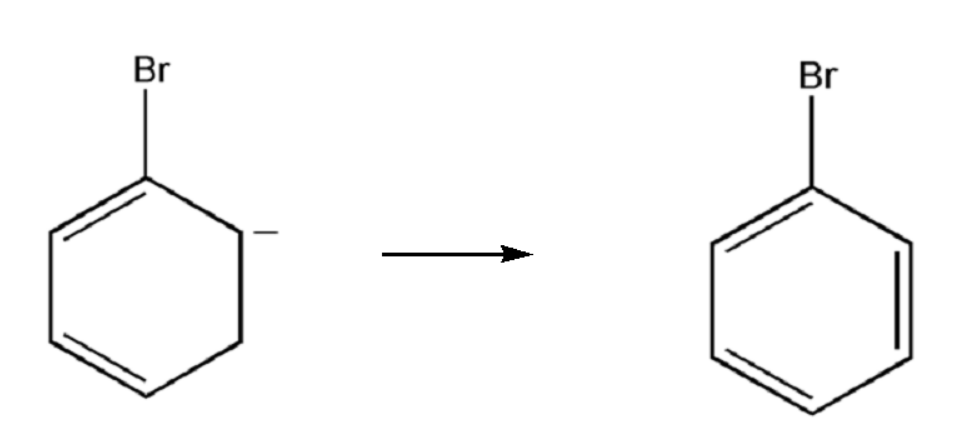
The rearrangement of the charge takes place and thus the final product we obtained is a benzene ring, which is a six member ring. Therefore the product of the above reaction is bromo-benzene.
Note:
Ring expansion takes place due to the stability of carbocation so formed during the reaction. It is always preferred to make six membered carbon rings than five membered carbon rings. This is because of less angle strain in the six member ring. Also the resonance takes place due to presence of double bonds at alternate positions. Hence we get bromo-benzene as the final product. While performing ring expansion it is mandatory to stabilize the carbocation so formed.
Complete Step By Step Answer:
When the bromoform reacts with the $ {\text{t - BuOK }} $ , it gives us the $ :CB{r_2} $ . Now $ :CB{r_2} $ will react with the given carbon ring. The reaction will proceed in the following steps as:
$ 1. $ $ :CB{r_2} $ thus formed will approach the carbon ring as,

$ 2. $ Now the ring chlorine atoms will leave the compound leaving behind the methyl group which will be attached to the ring by ring expansion.

$ 3. $ Now the cyclic compound will undergo ring expansion and hence it will form a six member ring. Bromine will leave the site and the methyl group gets bonded with the carbon of the ring.

$ 4. $ Now the stabilisation of charge takes place which in turn gives us a ring of benzene.

The rearrangement of the charge takes place and thus the final product we obtained is a benzene ring, which is a six member ring. Therefore the product of the above reaction is bromo-benzene.
Note:
Ring expansion takes place due to the stability of carbocation so formed during the reaction. It is always preferred to make six membered carbon rings than five membered carbon rings. This is because of less angle strain in the six member ring. Also the resonance takes place due to presence of double bonds at alternate positions. Hence we get bromo-benzene as the final product. While performing ring expansion it is mandatory to stabilize the carbocation so formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




