
Write the Lewis dot structure of $CO$ molecule.
Answer
550.2k+ views
Hint: Lewis structures are those in which the diagram shows the bonding between the atoms of a molecule and this also gives information of whether lone pairs of electrons exist or not. Based on this fact draw the dot structure of $CO$
Complete answer:
In the classes of chemistry, we have studied about writing the structure of a molecule based on various theories and have also come across the Lewis dot structures in the inorganic part of the chemistry.
Let us now write the electron dot structure also called as Lewis dot structure for $CO$molecule.
- Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
- Lewis structure shows each tom and its position in the structure of a molecule by making use of chemical symbols.
- Now, in the given molecule that is carbon monoxide with the chemical symbol $CO$, we have to follow several steps to write the Lewis dot structure.
- Firstly, count the total valence electron in this molecule.
We know that carbon has valence of 4 and oxygen has 6 valence electrons. By adding these two values, the total valence electrons present will be 10.
- Secondly, let us find the central atom. Here, the central atom is usually the atom with lowest electronegativity and in the molecule $CO$, carbon is less electronegative and thus is the central atom.
- Thirdly, draw the single bond to the central atom and put all the remaining valence electrons on atoms as a lone pair. Now for $CO$, the remaining electrons will be 8.
The structure here will be,
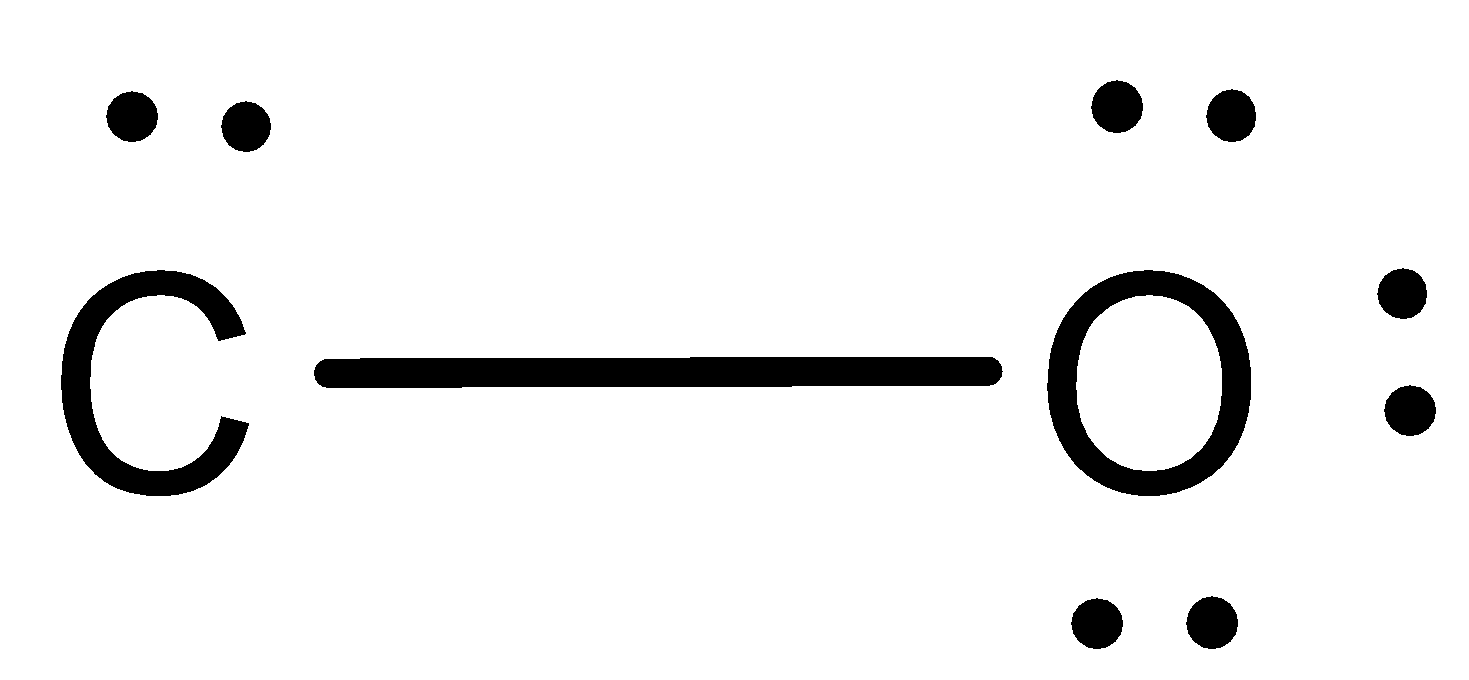
Now, since the valency of carbon is 4, we shall make it as having the 4 valency and by fulfilling the octet structure of oxygen. This can be done by drawing two more lines between carbon and oxygen. This will be,
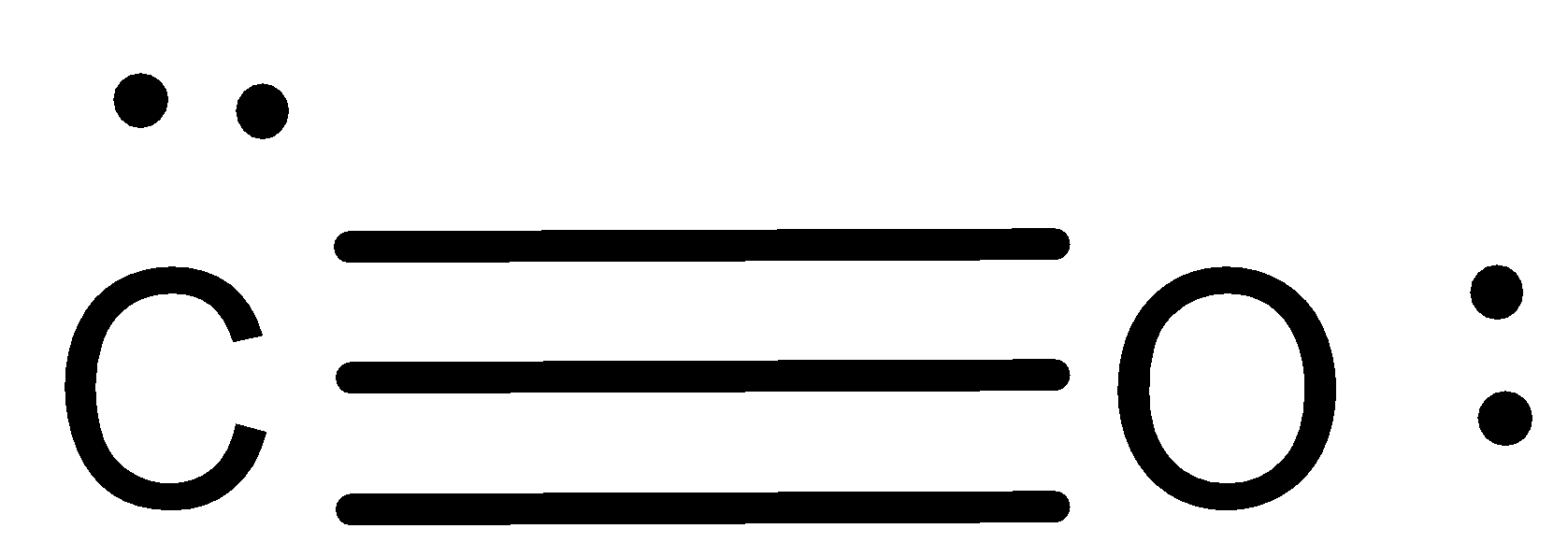
Now, this structure with the triple bond in between oxygen and carbon makes every atom with 8 valence electrons (octet) and thus this will be the Lewis dot structure for $CO$.
Note:
While writing the dot structure, be aware that whether the octet is complete for the ligands and fill up the octet of the atom which is not actually the central atom and it is of the atom which is attached to the central atom.
Complete answer:
In the classes of chemistry, we have studied about writing the structure of a molecule based on various theories and have also come across the Lewis dot structures in the inorganic part of the chemistry.
Let us now write the electron dot structure also called as Lewis dot structure for $CO$molecule.
- Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
- Lewis structure shows each tom and its position in the structure of a molecule by making use of chemical symbols.
- Now, in the given molecule that is carbon monoxide with the chemical symbol $CO$, we have to follow several steps to write the Lewis dot structure.
- Firstly, count the total valence electron in this molecule.
We know that carbon has valence of 4 and oxygen has 6 valence electrons. By adding these two values, the total valence electrons present will be 10.
- Secondly, let us find the central atom. Here, the central atom is usually the atom with lowest electronegativity and in the molecule $CO$, carbon is less electronegative and thus is the central atom.
- Thirdly, draw the single bond to the central atom and put all the remaining valence electrons on atoms as a lone pair. Now for $CO$, the remaining electrons will be 8.
The structure here will be,
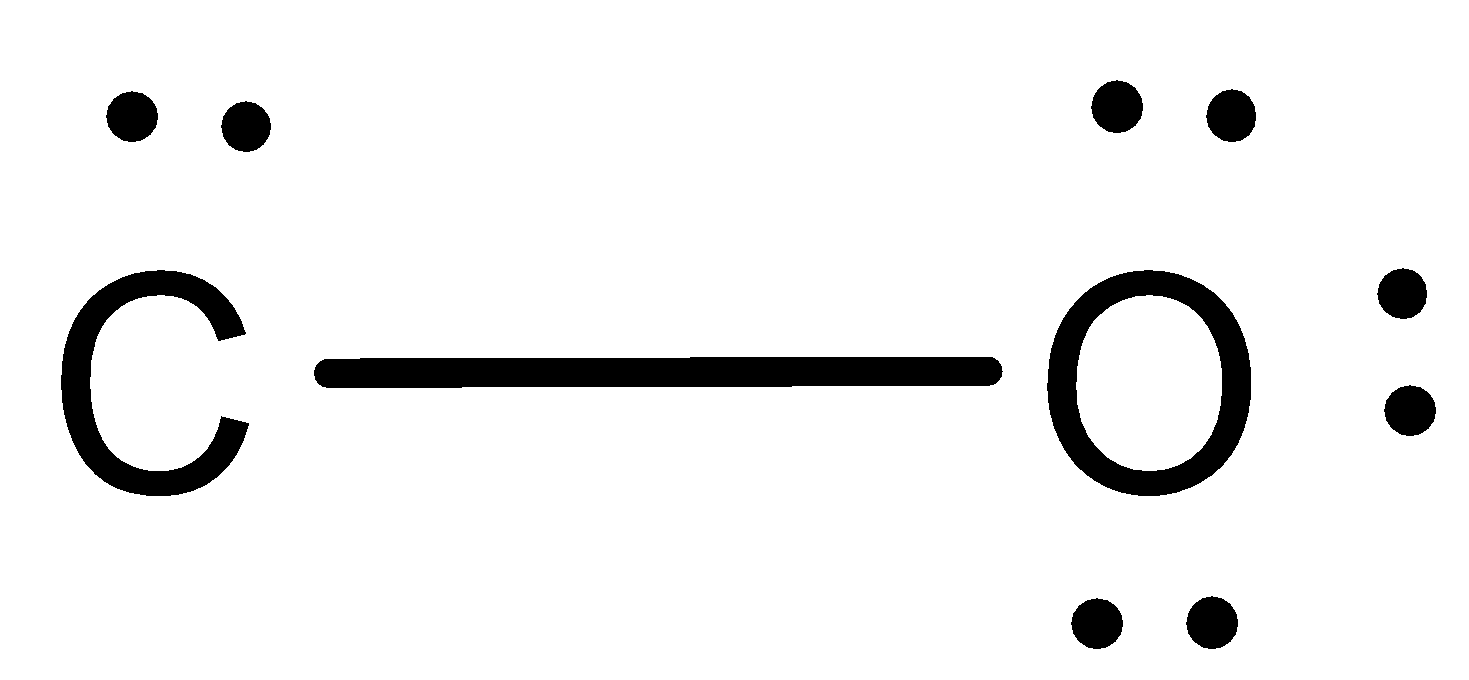
Now, since the valency of carbon is 4, we shall make it as having the 4 valency and by fulfilling the octet structure of oxygen. This can be done by drawing two more lines between carbon and oxygen. This will be,
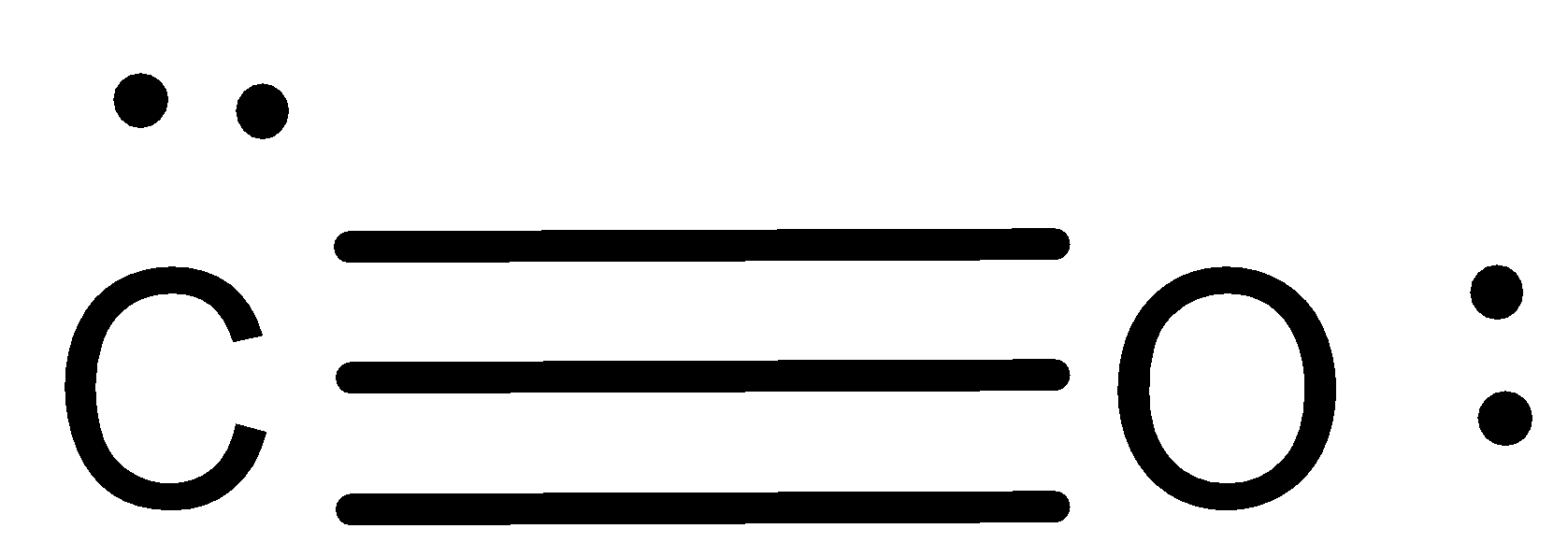
Now, this structure with the triple bond in between oxygen and carbon makes every atom with 8 valence electrons (octet) and thus this will be the Lewis dot structure for $CO$.
Note:
While writing the dot structure, be aware that whether the octet is complete for the ligands and fill up the octet of the atom which is not actually the central atom and it is of the atom which is attached to the central atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




