
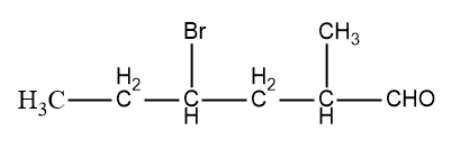
Write the IUPAC name of the following ketones or aldehyde. If possible, give a common name also.

Answer
570.3k+ views
Hint: To solve this question refer to the IUPAC Nomenclature which is basically a method of naming organic compounds, so, there are certain rules for that which we need to follow while writing the IUPAC name.
Complete answer:
We have been provided with a compound: $C{{H}_{3}}C{{H}_{2}}CH(Br)C{{H}_{2}}CH(C{{H}_ {3}}) CHO$,
We need to write its IUPAC names,
So, for that:
Firstly, the rules which are used to write the IUPAC name,
So, starting from choosing the longest carbon chain, this is called the parent chain.
Then we need to identify all the substituents present along the parent chain,
Then count the number of carbons from the end that would give the lowest number to the substituents,
If the substituent along the parent chain occurs more than once, then give the location of each substituent,
In case there are two or more different substituents they are arranged in the alphabetical order,
So, now the compound that we have: $C{{H}_{3}}C{{H}_{2}}CH(Br)C{{H}_{2}}CH(C{{H}_ {3}}) CHO$,
So, firstly we will choose the parent chain, after that count the number of carbons of the parent chain,
So, the number of carbons in parent chain is 6 and every bond is a single bond so it an alkane of 6 carbon that is hexane,
Now, we will move towards the substituents so on the fourth carbon bromo group is present, on second carbon a methyl group is present, also due to the presence of -CHO, it is an aldehyde,
Now, arranging them in alphabetical order,
So, the IUPAC name of the given compound comes out to be: 4-Bromo-2-methylhexanal,
Note:
If there is any cyclic hydrocarbon, it is designated by cyclo in front of the base name.
If chains of equal lengths are competing for selection of parent chain, then the chain which has the greatest number of side chains.
Complete answer:
We have been provided with a compound: $C{{H}_{3}}C{{H}_{2}}CH(Br)C{{H}_{2}}CH(C{{H}_ {3}}) CHO$,
We need to write its IUPAC names,
So, for that:
Firstly, the rules which are used to write the IUPAC name,
So, starting from choosing the longest carbon chain, this is called the parent chain.
Then we need to identify all the substituents present along the parent chain,
Then count the number of carbons from the end that would give the lowest number to the substituents,
If the substituent along the parent chain occurs more than once, then give the location of each substituent,
In case there are two or more different substituents they are arranged in the alphabetical order,
So, now the compound that we have: $C{{H}_{3}}C{{H}_{2}}CH(Br)C{{H}_{2}}CH(C{{H}_ {3}}) CHO$,
So, firstly we will choose the parent chain, after that count the number of carbons of the parent chain,
So, the number of carbons in parent chain is 6 and every bond is a single bond so it an alkane of 6 carbon that is hexane,
Now, we will move towards the substituents so on the fourth carbon bromo group is present, on second carbon a methyl group is present, also due to the presence of -CHO, it is an aldehyde,
Now, arranging them in alphabetical order,
So, the IUPAC name of the given compound comes out to be: 4-Bromo-2-methylhexanal,
Note:
If there is any cyclic hydrocarbon, it is designated by cyclo in front of the base name.
If chains of equal lengths are competing for selection of parent chain, then the chain which has the greatest number of side chains.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




