
Write the IUPAC name of the following compounds:
(a)-
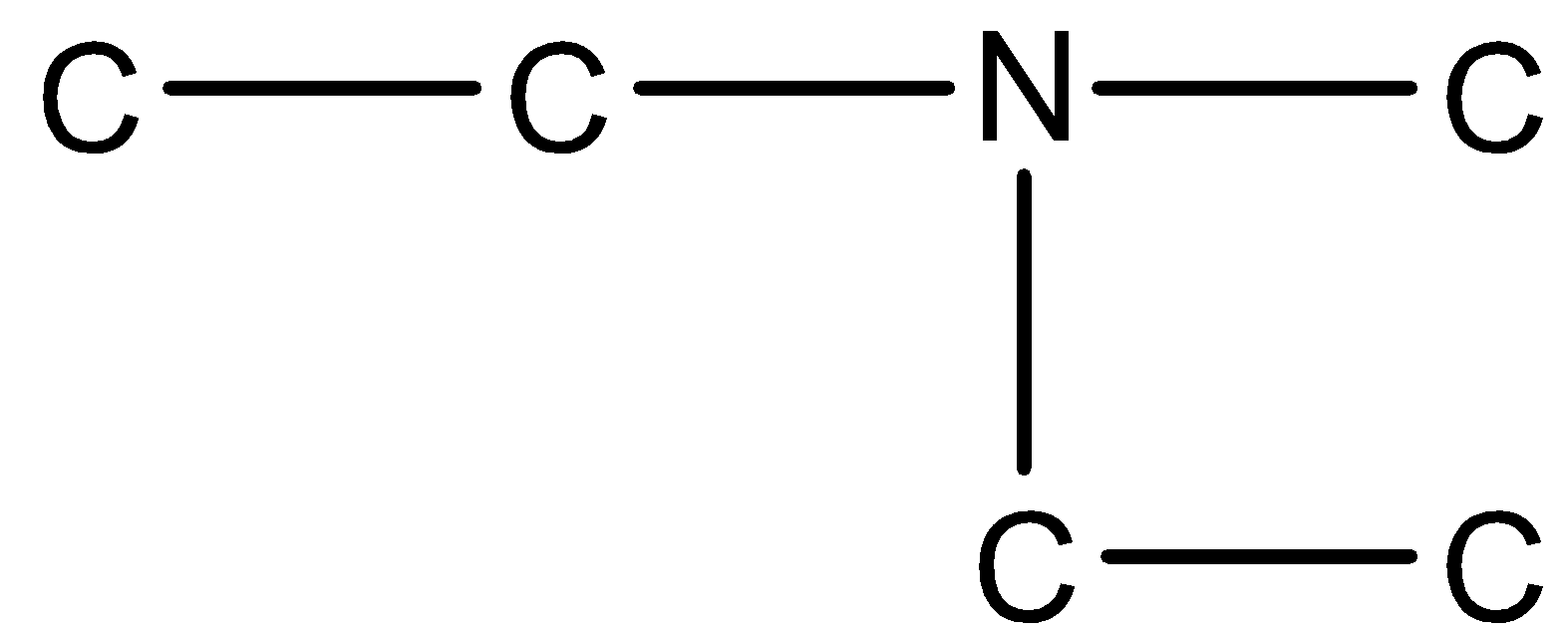
(b)-
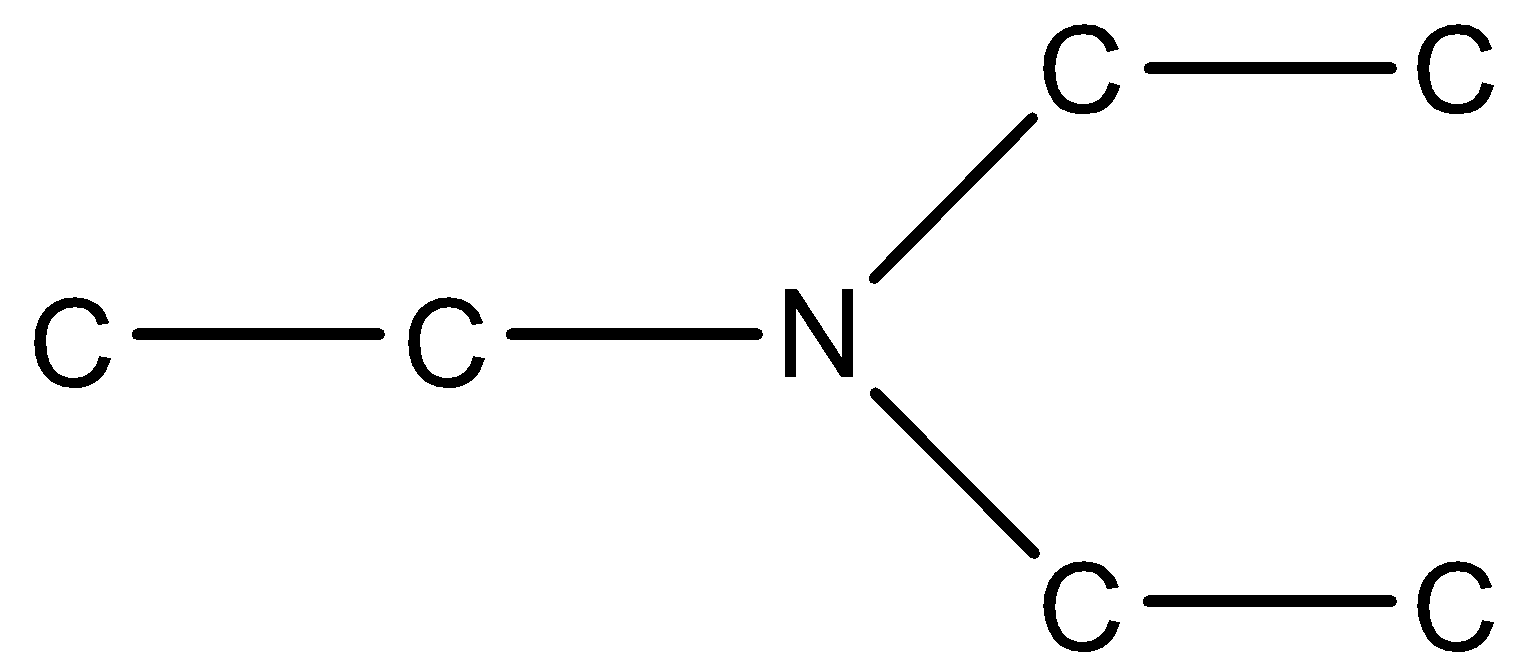


Answer
531.9k+ views
Hint: In both the compounds there is nitrogen and nitrogen is present as amine, so both the compounds are amines. If the chain of the carbon atoms is two then use ethyl term and if the chain of carbon atom is one then use methyl term.
Complete answer:
The given two compounds in the question are organic compounds in which the functional group is present as nitrogen atoms and the nitrogen atom is present as amine. In both the compounds, there are three different carbon chains present, as there is no hydrogen atom present to the nitrogen atom, therefore, both the compounds are tertiary amines.
In option (a), the three chains that are present in the nitrogen atoms are two ethyl groups and one methyl group. Therefore, the largest chain is of the ethyl group. In the IUPAC name of the following compound, we have to first the substituent groups of the amine group and add N to the chain. The naming should be done in alphabetic order.
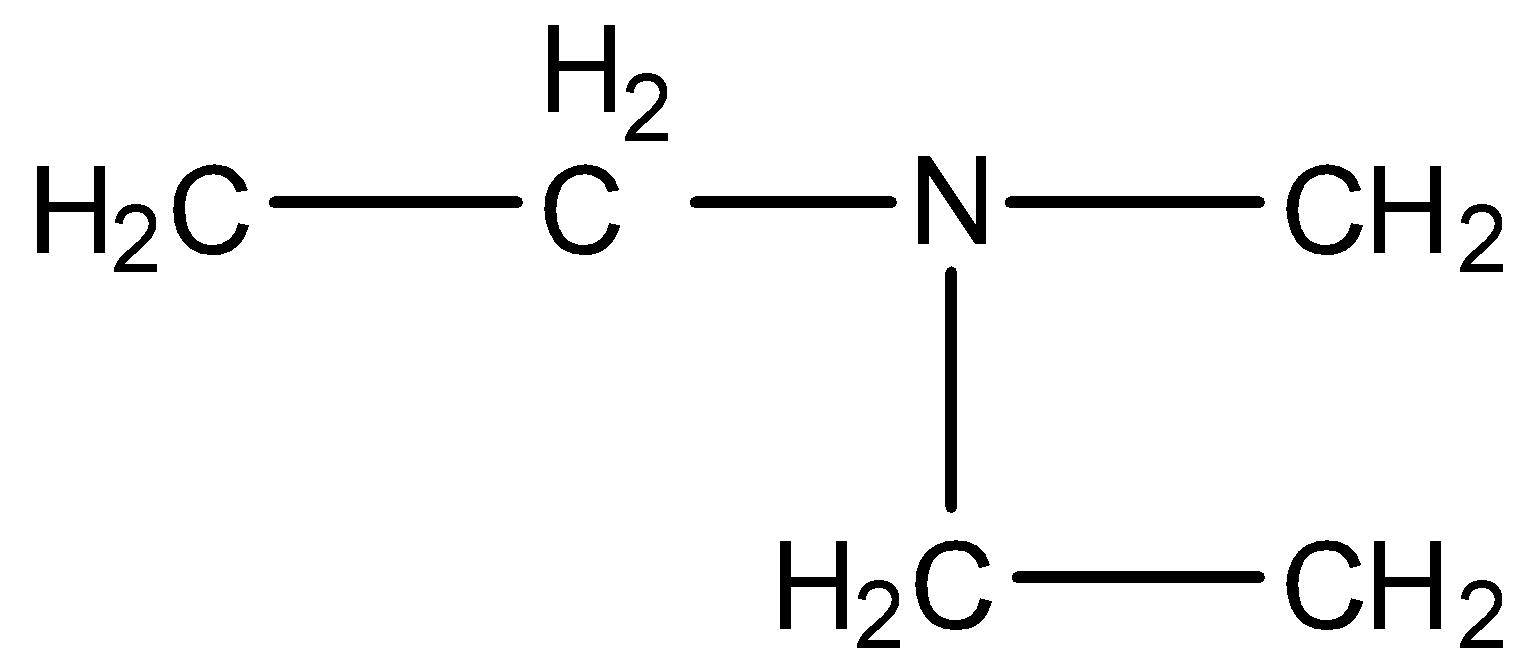
In these two substituent chains are ethyl and methyl, alphabetically e comes before m, and the main chain will be ethanamine.
Therefore, its name will be N-Ethyl-N-methyl ethanamine.
In option (b), the three chains that are present to the nitrogen atoms are three ethyl groups. Therefore, the largest chain is of the ethyl group.
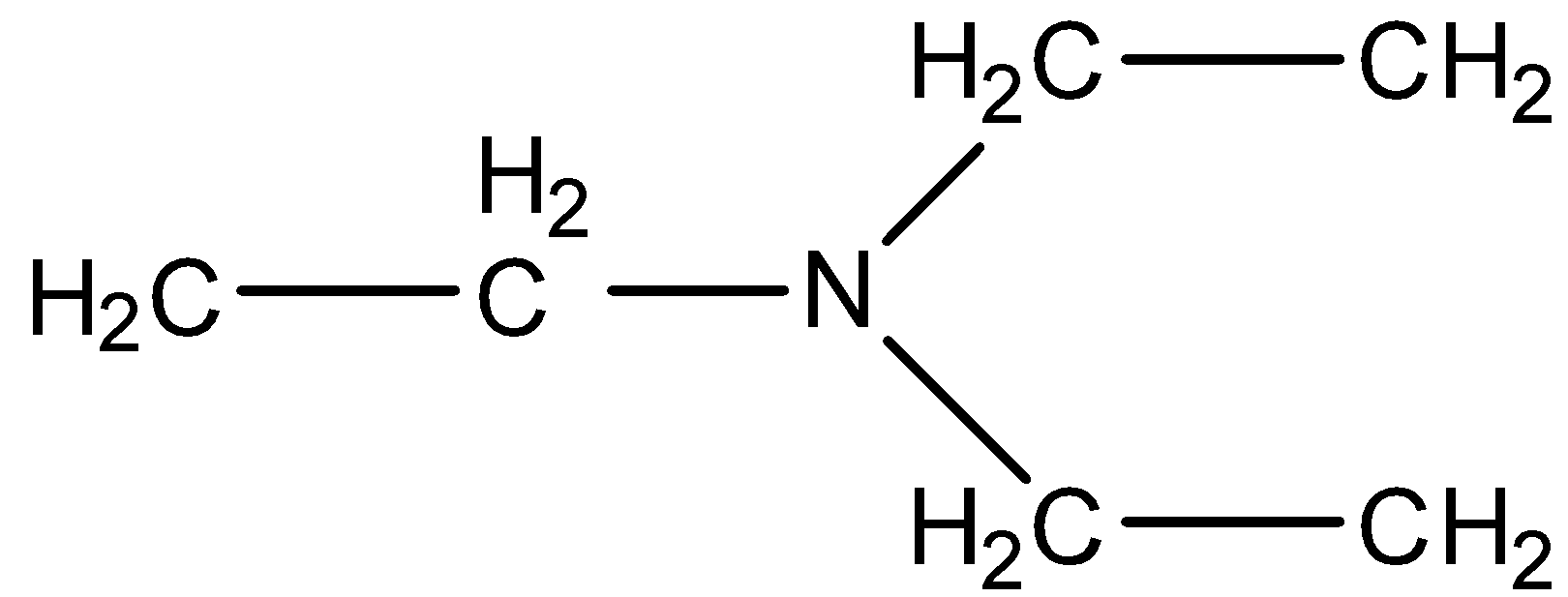
In these two substituent chains are ethyl groups, so we can use di in the prefix and the main chain will be ethanamine.
Therefore, its name will be N, N-Diethyl ethanamine.
Note:
Whenever we name secondary and tertiary amines, don’t forget to use N before the substituent carbon chains. If three carbon atoms chain is present then use propyl term, if four carbon atoms chain are present then use butyl term, etc.
Complete answer:
The given two compounds in the question are organic compounds in which the functional group is present as nitrogen atoms and the nitrogen atom is present as amine. In both the compounds, there are three different carbon chains present, as there is no hydrogen atom present to the nitrogen atom, therefore, both the compounds are tertiary amines.
In option (a), the three chains that are present in the nitrogen atoms are two ethyl groups and one methyl group. Therefore, the largest chain is of the ethyl group. In the IUPAC name of the following compound, we have to first the substituent groups of the amine group and add N to the chain. The naming should be done in alphabetic order.

In these two substituent chains are ethyl and methyl, alphabetically e comes before m, and the main chain will be ethanamine.
Therefore, its name will be N-Ethyl-N-methyl ethanamine.
In option (b), the three chains that are present to the nitrogen atoms are three ethyl groups. Therefore, the largest chain is of the ethyl group.

In these two substituent chains are ethyl groups, so we can use di in the prefix and the main chain will be ethanamine.
Therefore, its name will be N, N-Diethyl ethanamine.
Note:
Whenever we name secondary and tertiary amines, don’t forget to use N before the substituent carbon chains. If three carbon atoms chain is present then use propyl term, if four carbon atoms chain are present then use butyl term, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




