
Write the IUPAC name of the following:
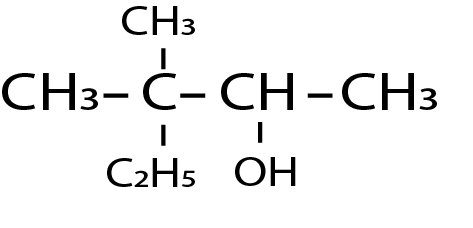
Answer
542.4k+ views
Hint: Functional group: In the hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound. And if hydroxide group i.e. $OH$ is present then the name will be alcohol.
Complete answer:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound. And if hydroxide group i.e. $OH$ is present then the name will be alcohol.
The first step in the IUPAC naming system is to identify the longest carbon chain containing the groups. Here in the given compound the largest chain contains five carbons. So the name of the parent chain will be pentane. Now the numbering of the carbon is done such that carbon which contains function gets a lower number. Here the functional group attached the hydrocarbon is alcohol. So numbering of carbon should be done from right to left. And the second carbon alcohol group is present and to the third carbon two methyl groups are present. So the IUPAC name of the molecule will be as $3,3 - $ methyl pentan $2 - $ ol.
Note:
Suffix to some functional group are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol or in place of e from alkane add ol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Complete answer:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
| Number of carbon atom in alkane | Name of the parent chain |
| One | Methane |
| Two | Ethane |
| Three | Propane |
| Four | Butane |
| Five | Pentane |
| Six | Hexane |
| Seven | Heptane |
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound. And if hydroxide group i.e. $OH$ is present then the name will be alcohol.
The first step in the IUPAC naming system is to identify the longest carbon chain containing the groups. Here in the given compound the largest chain contains five carbons. So the name of the parent chain will be pentane. Now the numbering of the carbon is done such that carbon which contains function gets a lower number. Here the functional group attached the hydrocarbon is alcohol. So numbering of carbon should be done from right to left. And the second carbon alcohol group is present and to the third carbon two methyl groups are present. So the IUPAC name of the molecule will be as $3,3 - $ methyl pentan $2 - $ ol.
Note:
Suffix to some functional group are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol or in place of e from alkane add ol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




