
Write the IUPAC name of phthalic acid?
Answer
513k+ views
Hint :Note that while naming substituted benzene compounds, we prefix the name of the substituent to the word benzene. In the case of mono-substituted benzene, we prefix the name of the substituent to benzene simply. In the case of di-substituted benzene, we have to number each of the carbon atoms in such a way that the substituents are attached to the lowest possible numbered carbon.
Complete Step By Step Answer:
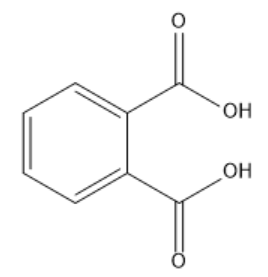
In phthalic acid, there are two carboxylic groups attached to the benzene ring. Here, the two carboxylic groups occupy positions next to each other in the benzene ring. So, it is ortho substitution. Carboxylic acid functional group has higher priority, so start the numbering from any of the carboxylic acid groups. Then continue numbering in a way that the carbon attached to the second carboxylic acid group gets a less number. So, both the carboxylic acids get numbers one and two. So, the IUPAC name of phthalic acid will be ${\text{Benzene - 1,2 - dicarboxylic acid}}$. Here, we used prefix di because there are two carboxylic acid groups in the given compound. The structure of phthalic acid is given below:
Note :
If there are multiple substituents on the ring, remember to number the carbons of the ring in such a way that the carbons with substituents have the lowest possible number. Note that the substituents must be placed in alphabetical order while naming. If there is only one substituent on the ring then the ring carbon that is attached to the substituent will get number one.
Complete Step By Step Answer:
In phthalic acid, there are two carboxylic groups attached to the benzene ring. Here, the two carboxylic groups occupy positions next to each other in the benzene ring. So, it is ortho substitution. Carboxylic acid functional group has higher priority, so start the numbering from any of the carboxylic acid groups. Then continue numbering in a way that the carbon attached to the second carboxylic acid group gets a less number. So, both the carboxylic acids get numbers one and two. So, the IUPAC name of phthalic acid will be ${\text{Benzene - 1,2 - dicarboxylic acid}}$. Here, we used prefix di because there are two carboxylic acid groups in the given compound. The structure of phthalic acid is given below:

Note :
If there are multiple substituents on the ring, remember to number the carbons of the ring in such a way that the carbons with substituents have the lowest possible number. Note that the substituents must be placed in alphabetical order while naming. If there is only one substituent on the ring then the ring carbon that is attached to the substituent will get number one.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




