
Write the IUPAC name of ${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$ ion and draw the structure of its geometrical isomer.
Answer
585.3k+ views
Hint: We know the,
Rules for naming the complex:
-First, name the ligand and then the metal atom or ion. A Roman numeral in parentheses denotes the oxidation number of the central metal ion.
-Neutral ligands, such as $H_2NCH_2CH_2NH_2$ (ethylenediamine) have the same name as the molecule, except for \[{{\text{H}}_{\text{2}}}{\text{O}}\] (aqua),\[{\text{N}}{{\text{H}}_{\text{3}}}\] (ammine), \[{\text{CO}}\] (carbonyl), and \[{\text{NO}}\] (nitrosyl).
-Anionic ligands end in -o; for anions that end in -ide (such as chloride), -ate (such as sulfate), and -ite (such as nitrite), change the endings as -ide, -ido, ate, -ato, ite, -ito.
Examples: chlorido, sulfato, and nitrito.
-The number of each type of ligand in the complex ion has to be indicated by Greek prefixes.
-If the ligand already contains a Greek prefix (such as the diethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the following prefixes are used instead 2-bis, 3-tris, 4-tetrakis.
-Ligands are named in alphabetical order, ignoring any Greek prefix.
-If there is an uncertainty in identifying which atom is linked to the metal atom, then k E is added to the name in parentheses, where E denotes the connecting atom (and k is kappa).
-If the overall charge of the complex is negative (an anionic complex), the suffix -ate is added to the stem of the metal’s name. If the symbol of the metal originates from a Latin name, then the Latin stem is used followed by the oxidation number of the metal in Roman numerals.
-The name of a coordination compound (as distinct from a complex cation or anion) is built in the same way as that of a simple compound, with the cation named before the anion.
Complete step by step answer:
The given complex is ${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$,
-The complex has two ligands that ethylene diamine and the chloride, if there is more than one ligand then the ligand is named as in the alphabetical order thus ‘chlorine’ ligand is named first since there is two ligands it is denoted by the Greek prefix ‘di’.
-Since the ethylene diamine ligand already contains a Greek prefix (such as the diethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the following prefixes are used instead 2-bis.
-If the anionic ligand name ends in ‘ide’ the ending must be changed as ‘ido’ thus chloride ligand is named as chlorido.
-After naming the ligands in the parentheses, the metal must be named with its oxidation number in roman numerals. The oxidation number of cobalt in the complex is$ + 3$ .
The systematic name of ${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$complex is,\[Dichlorobis\left( {ethylenediamine} \right){\text{ }}Cobalt{\text{ }}\left( {III} \right){\text{ }}Chloride\]
The structures of cis and Trans isomers are,
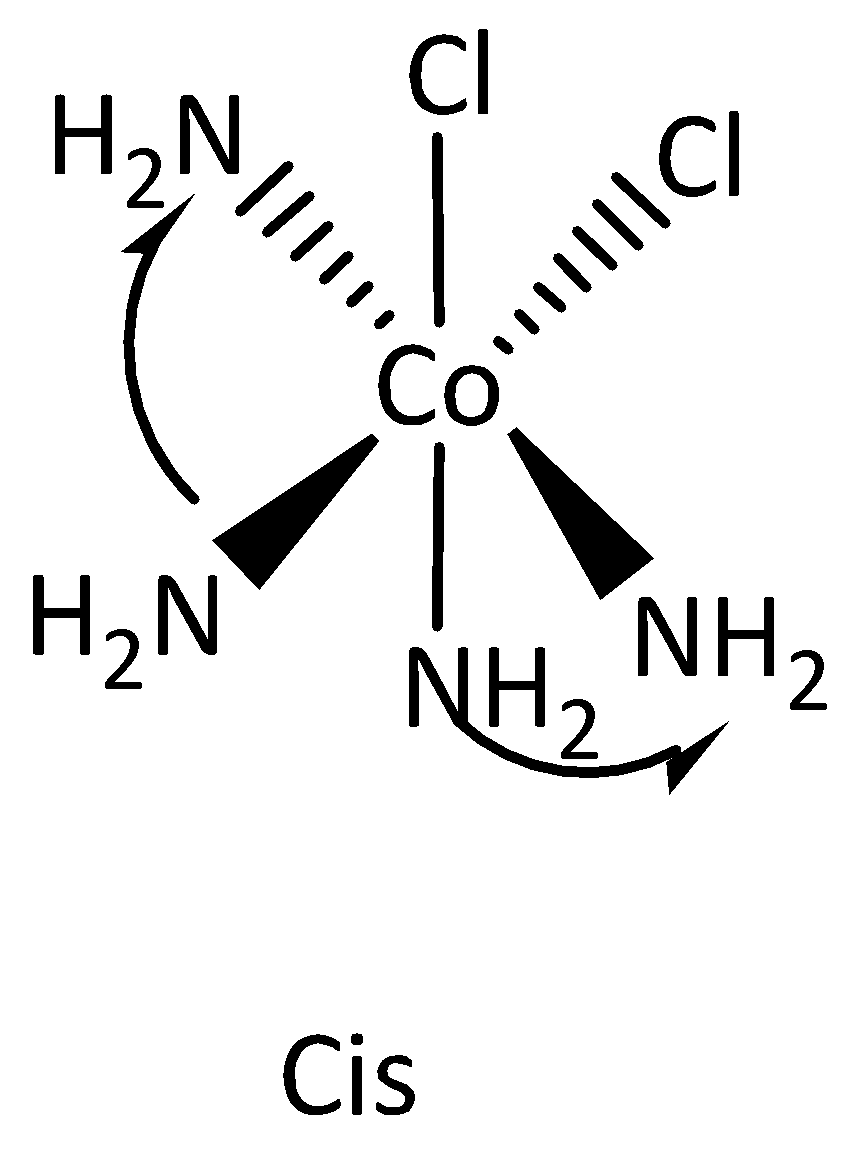
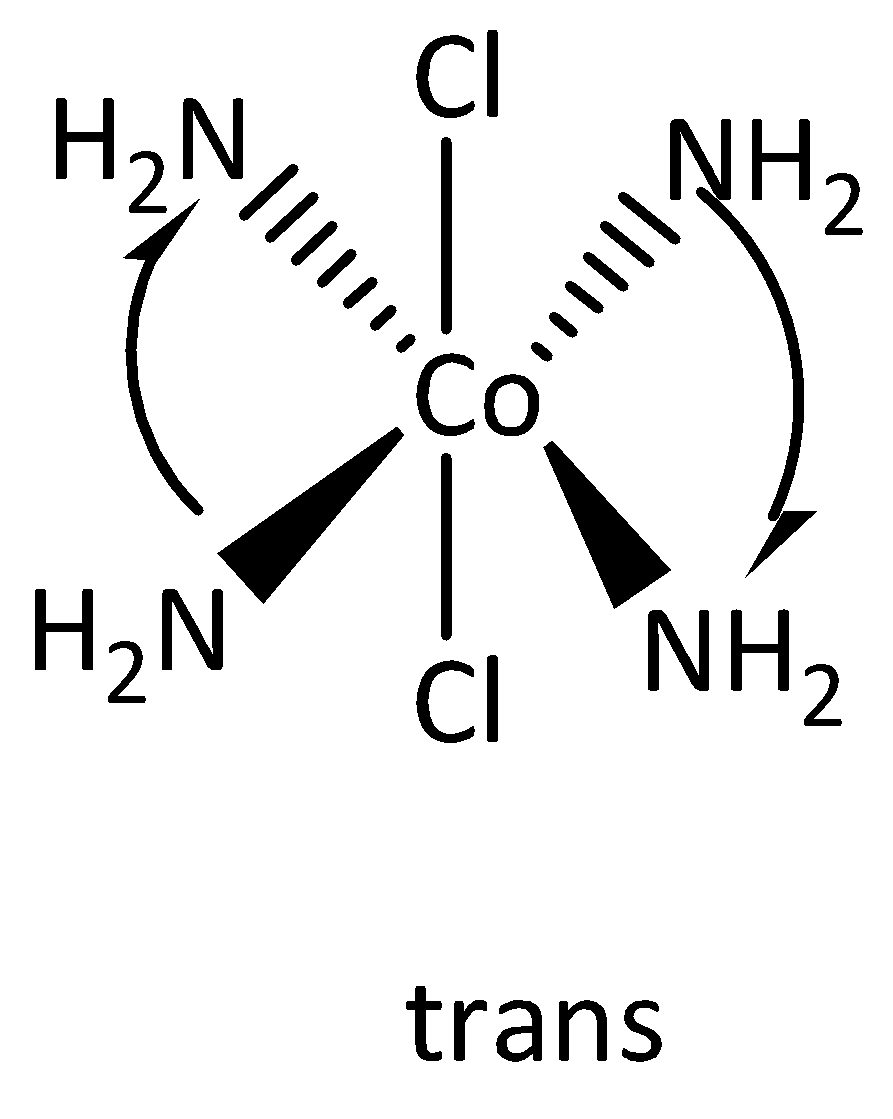
Note:
Now we discuss the details of Geometric isomers.
Geometric isomers are isomers which arise due to different arrangements of atoms or groups around a bond which has restricted rotation. Geometric isomers are also known as Cis- Tran’s isomerism.
-If the two atoms locked in same side of the molecule then it is called as cis isomers.
-If the two atoms are locked on opposite sides of the molecule then it is called Trans isomers.
Rules for naming the complex:
-First, name the ligand and then the metal atom or ion. A Roman numeral in parentheses denotes the oxidation number of the central metal ion.
-Neutral ligands, such as $H_2NCH_2CH_2NH_2$ (ethylenediamine) have the same name as the molecule, except for \[{{\text{H}}_{\text{2}}}{\text{O}}\] (aqua),\[{\text{N}}{{\text{H}}_{\text{3}}}\] (ammine), \[{\text{CO}}\] (carbonyl), and \[{\text{NO}}\] (nitrosyl).
-Anionic ligands end in -o; for anions that end in -ide (such as chloride), -ate (such as sulfate), and -ite (such as nitrite), change the endings as -ide, -ido, ate, -ato, ite, -ito.
Examples: chlorido, sulfato, and nitrito.
-The number of each type of ligand in the complex ion has to be indicated by Greek prefixes.
-If the ligand already contains a Greek prefix (such as the diethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the following prefixes are used instead 2-bis, 3-tris, 4-tetrakis.
-Ligands are named in alphabetical order, ignoring any Greek prefix.
-If there is an uncertainty in identifying which atom is linked to the metal atom, then k E is added to the name in parentheses, where E denotes the connecting atom (and k is kappa).
-If the overall charge of the complex is negative (an anionic complex), the suffix -ate is added to the stem of the metal’s name. If the symbol of the metal originates from a Latin name, then the Latin stem is used followed by the oxidation number of the metal in Roman numerals.
-The name of a coordination compound (as distinct from a complex cation or anion) is built in the same way as that of a simple compound, with the cation named before the anion.
Complete step by step answer:
The given complex is ${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$,
-The complex has two ligands that ethylene diamine and the chloride, if there is more than one ligand then the ligand is named as in the alphabetical order thus ‘chlorine’ ligand is named first since there is two ligands it is denoted by the Greek prefix ‘di’.
-Since the ethylene diamine ligand already contains a Greek prefix (such as the diethylenediamine) or if it is polydentate (able to attach at more than one binding site simultaneously), then the following prefixes are used instead 2-bis.
-If the anionic ligand name ends in ‘ide’ the ending must be changed as ‘ido’ thus chloride ligand is named as chlorido.
-After naming the ligands in the parentheses, the metal must be named with its oxidation number in roman numerals. The oxidation number of cobalt in the complex is$ + 3$ .
The systematic name of ${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$complex is,\[Dichlorobis\left( {ethylenediamine} \right){\text{ }}Cobalt{\text{ }}\left( {III} \right){\text{ }}Chloride\]
The structures of cis and Trans isomers are,


Note:
Now we discuss the details of Geometric isomers.
Geometric isomers are isomers which arise due to different arrangements of atoms or groups around a bond which has restricted rotation. Geometric isomers are also known as Cis- Tran’s isomerism.
-If the two atoms locked in same side of the molecule then it is called as cis isomers.
-If the two atoms are locked on opposite sides of the molecule then it is called Trans isomers.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




