
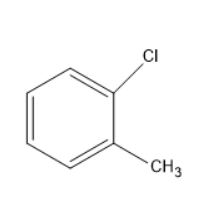
Write the IUPAC name of

Answer
501.3k+ views
Hint: Benzene is an aromatic compound and the presence of methyl substituent on benzene ring made the molecule as methyl benzene and the common name is toluene. The substituent present on toluene is chlorine atom. Thus, the position and name of substituent must be written before the benzene ring.
Complete answer:
Benzene is a chemical compound with molecular formula $ {C_6}{H_6} $ , and is an aromatic compound. As it is cyclic, planar, conjugation of $ \pi $ electrons and obeying Huckel's rule. Huckel’s rule states that the compound must contain $ \left( {4n + 2} \right)\pi $ electrons, where n is a whole number. Here benzene contains $ 6\pi $ electrons.
The name of the compound with methyl substituent on the benzene ring can be called toluene. The chlorine atom is substituted on the toluene then the molecule can be called as chloro toluene.
While writing the IUPAC nomenclature, the position of substituent must also be considered. The substituent must also get the lowest number.
The numbering should start from methyl substituent on benzene ring and the numbering should be done in an anti-clockwise direction in the given molecule to get the lowest numbering for substituent.
Thus, the IUPAC name of the given compound will be $ 2 - $ chloro toluene.
Note:
The methyl substituent on the benzene ring makes the molecule more attractive towards the electrophile as the methyl group is an electron releasing group. Here chlorination of methyl benzene is also an example of electrophilic substitution as the chlorine atom is substituted in the place of hydrogen.
Complete answer:
Benzene is a chemical compound with molecular formula $ {C_6}{H_6} $ , and is an aromatic compound. As it is cyclic, planar, conjugation of $ \pi $ electrons and obeying Huckel's rule. Huckel’s rule states that the compound must contain $ \left( {4n + 2} \right)\pi $ electrons, where n is a whole number. Here benzene contains $ 6\pi $ electrons.
The name of the compound with methyl substituent on the benzene ring can be called toluene. The chlorine atom is substituted on the toluene then the molecule can be called as chloro toluene.
While writing the IUPAC nomenclature, the position of substituent must also be considered. The substituent must also get the lowest number.
The numbering should start from methyl substituent on benzene ring and the numbering should be done in an anti-clockwise direction in the given molecule to get the lowest numbering for substituent.
Thus, the IUPAC name of the given compound will be $ 2 - $ chloro toluene.
Note:
The methyl substituent on the benzene ring makes the molecule more attractive towards the electrophile as the methyl group is an electron releasing group. Here chlorination of methyl benzene is also an example of electrophilic substitution as the chlorine atom is substituted in the place of hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




