
Write the formula of Pyrosulphuric acid:
$
A){H_2}{S_2}{O_7} \\
B){H_2}S{O_5} \\
C){H_2}{S_2}{O_8} \\
D)None\,of\,these \\
$
Answer
590.1k+ views
Hint: We know that disulfuric acid is otherwise called as pyrosulfuric acid. It is an example of sulfur oxoacid. The salts of disulfur acids are pyrosulfates. An example of pyrosulfate is potassium pyrosulfate.
Complete step by step answer:
We have to know that the IUPAC name of pyrosulfuric acid is disulfuric acid. Oleum is the other name given to pyrosulfuric acid.
In pyrosulfuric acid, there are two atoms of sulfur, seven atoms of oxygen and two atoms of hydrogens. We can draw the structure of pyrosulfuric acid,
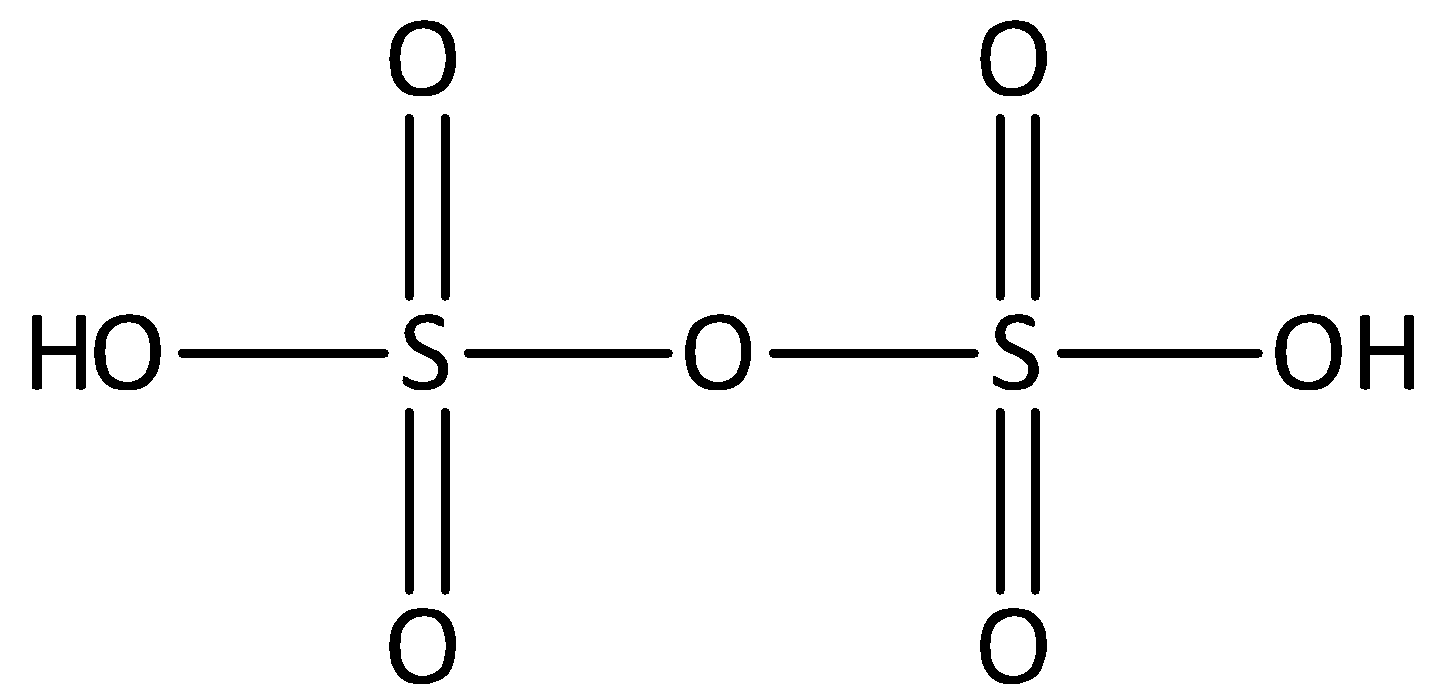
From the given structure, we can see that each sulfur atom is doubled bonded to two oxygen atoms and single bonded to other oxygen atoms. The oxidation state of sulfur in pyrosulfuric acid is ${\text{ + 6}}{\text{.}}$
We can prepare pyrosulfuric acid by the dissolution of fumes of sulfur trioxide in sulfuric acid.
We can write the chemical reaction as,
${H_2}S{O_4} + S{O_3}\xrightarrow{{}}{H_2}{S_2}{O_7}$
The chemical formula of pyrosulfuric acid is ${H_2}{S_2}{O_7}.$ Therefore, Option (A) is correct.
The compound that has a chemical formula of ${H_2}S{O_5}$ is peroxymonosulfuric acid. It is also an oxyacid of sulfur. Option (B) is incorrect.
The chemical formula ${H_2}{S_2}{O_8}$ represents peroxydisulfuric acid. It is also an oxoacid of sulfur.
Hence option (C) is correct.
Note:
We can write the IUPAC name of peroxymonosulfuric acid as peroxydisulfuric acid (or) sulfurperoxic acid. Peroxymonosulfuric acid is used as disinfectant and cleaning in swimming pools. Salts of peroxymonosulfuric acid are used in the plastic industry and for decolorizing oils.
We can write the IUPAC name of peroxydisulfuric acid as persulfuric acid. It Is also known as Marshall’s acid.
Complete step by step answer:
We have to know that the IUPAC name of pyrosulfuric acid is disulfuric acid. Oleum is the other name given to pyrosulfuric acid.
In pyrosulfuric acid, there are two atoms of sulfur, seven atoms of oxygen and two atoms of hydrogens. We can draw the structure of pyrosulfuric acid,

From the given structure, we can see that each sulfur atom is doubled bonded to two oxygen atoms and single bonded to other oxygen atoms. The oxidation state of sulfur in pyrosulfuric acid is ${\text{ + 6}}{\text{.}}$
We can prepare pyrosulfuric acid by the dissolution of fumes of sulfur trioxide in sulfuric acid.
We can write the chemical reaction as,
${H_2}S{O_4} + S{O_3}\xrightarrow{{}}{H_2}{S_2}{O_7}$
The chemical formula of pyrosulfuric acid is ${H_2}{S_2}{O_7}.$ Therefore, Option (A) is correct.
The compound that has a chemical formula of ${H_2}S{O_5}$ is peroxymonosulfuric acid. It is also an oxyacid of sulfur. Option (B) is incorrect.
The chemical formula ${H_2}{S_2}{O_8}$ represents peroxydisulfuric acid. It is also an oxoacid of sulfur.
Hence option (C) is correct.
Note:
We can write the IUPAC name of peroxymonosulfuric acid as peroxydisulfuric acid (or) sulfurperoxic acid. Peroxymonosulfuric acid is used as disinfectant and cleaning in swimming pools. Salts of peroxymonosulfuric acid are used in the plastic industry and for decolorizing oils.
We can write the IUPAC name of peroxydisulfuric acid as persulfuric acid. It Is also known as Marshall’s acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




