
How do you write the formula of periodic acid?
Answer
558.6k+ views
Hint: Periodic acid is the oxoacid of the iodine. The iodine in the periodic acid has an oxidation state ${\text{ + 7}}$ and consists of four oxygen atoms in its structure. One can try to predict the structure and molecular formula of periodic acid based on this information.
Complete step by step answer:
1) First of all, we will understand the chemical compound periodic acid. Periodic acid consists of iodine and it is the highest oxoacid of iodine. The oxoacid can be defined as the element which has oxygen, hydrogen, and another element in it.
2) Now as the periodic acid is an oxoacid of iodine, the element iodine has an ${\text{ + 7}}$ oxidation state in it which means there are three oxygen atoms that are double bonded to the central iodine atom and one hydroxyl group which is single bonded to the central atom.
3) Now let us see the structure of periodic acid as below,
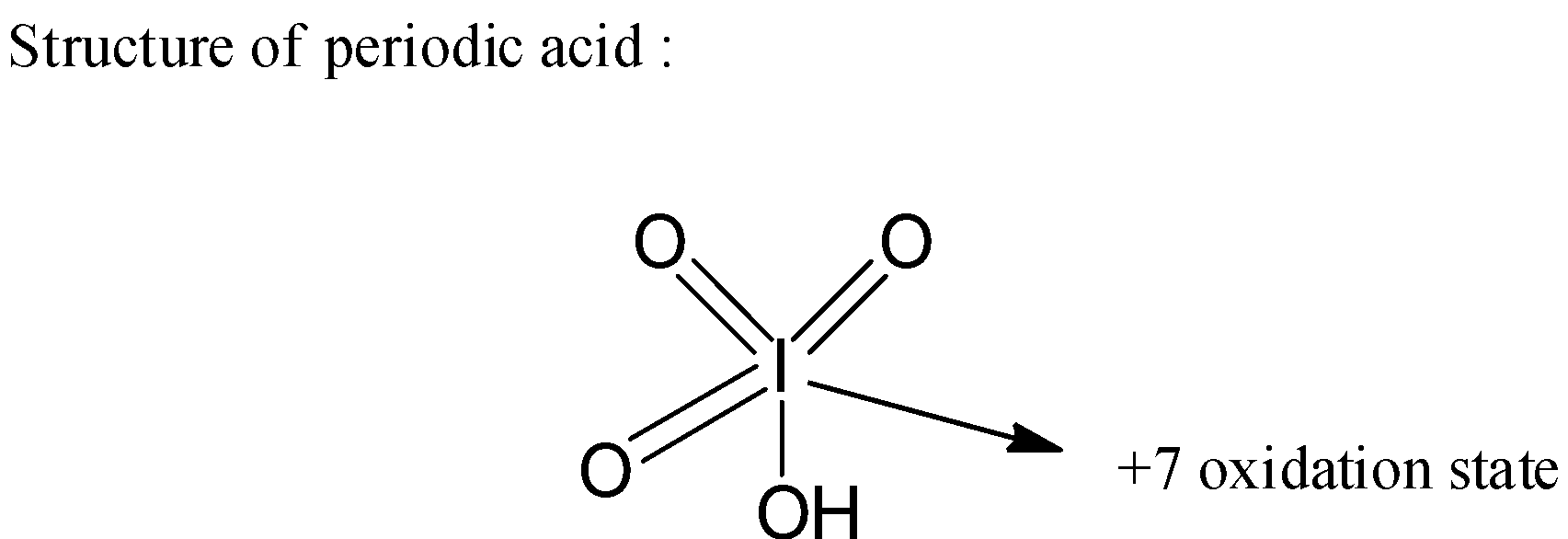
4) Now if we observe the above structure the ${\text{ + 7}}$ oxidation state of the central iodine atom is due to surrounding three double bonded oxygens which has ${\text{ - 2}}$ oxidation state each and one ${\text{ - OH}}$ group which has an oxidation state as ${\text{ - 1}}$.
Therefore, we can write the formula of the periodic acid as $HI{O_4}$.
Note:
The periodic acid is a periodate that means the structure can exist in two forms such as orthoperiodic acid which has a chemical formula as ${H_5}I{O_6}$ and meta periodic acid which has a chemical formula as $HI{O_4}$. Periodic acid is soluble in water and alcohols and has a melting point of ${\text{128}} \cdot {{\text{5}}^o}C$.
Complete step by step answer:
1) First of all, we will understand the chemical compound periodic acid. Periodic acid consists of iodine and it is the highest oxoacid of iodine. The oxoacid can be defined as the element which has oxygen, hydrogen, and another element in it.
2) Now as the periodic acid is an oxoacid of iodine, the element iodine has an ${\text{ + 7}}$ oxidation state in it which means there are three oxygen atoms that are double bonded to the central iodine atom and one hydroxyl group which is single bonded to the central atom.
3) Now let us see the structure of periodic acid as below,

4) Now if we observe the above structure the ${\text{ + 7}}$ oxidation state of the central iodine atom is due to surrounding three double bonded oxygens which has ${\text{ - 2}}$ oxidation state each and one ${\text{ - OH}}$ group which has an oxidation state as ${\text{ - 1}}$.
Therefore, we can write the formula of the periodic acid as $HI{O_4}$.
Note:
The periodic acid is a periodate that means the structure can exist in two forms such as orthoperiodic acid which has a chemical formula as ${H_5}I{O_6}$ and meta periodic acid which has a chemical formula as $HI{O_4}$. Periodic acid is soluble in water and alcohols and has a melting point of ${\text{128}} \cdot {{\text{5}}^o}C$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




