
Write the formula of any two oxides of sulphur.
Answer
579.9k+ views
Hint: Sulphur is an abundantly found non-metal. It is odorless and tasteless. Sulphur is a yellow crystalline solid in its native form. It occurs as a pure element, as sulphate or sulphide minerals.
Complete solution:
There are several oxides of sulphur. The two most important oxides of sulphur are sulphur dioxide and sulphur trioxide.
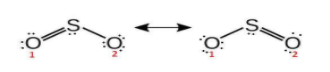
Sulphur dioxide( $SO_2$) : Naturally, sulphur dioxide is released by volcanic activity. It is produced by
burning of fossil fuel that is contaminated with sulphur components and also obtained as a by-product during copper extraction. The bond order of sulphur-oxygen is 1.5. The two resonance structure of sulphur dioxide is
Sulphur trioxide ( $SO_3$ ): It is relatively a narrow liquid. In gaseous form it is a major pollutant that contributes to acid rain. It is a precursor to sulphuric acid and also called sulphuric anhydride. The bond order is 1.33. The vapour form of sulphur trioxide is invisible. It is transparent in liquid form. The resonance structure of sulphur trioxide is

Note:
Inhalation of sulphur dioxide and sulphur trioxide affects the respiratory tract which causes lung infection, irritation of eyes and in severe cases it causes asthma and chronic bronchitis. When these oxides come in contact with the organic materials, they are inflammable.
Complete solution:
There are several oxides of sulphur. The two most important oxides of sulphur are sulphur dioxide and sulphur trioxide.
Sulphur dioxide( $SO_2$) : Naturally, sulphur dioxide is released by volcanic activity. It is produced by
burning of fossil fuel that is contaminated with sulphur components and also obtained as a by-product during copper extraction. The bond order of sulphur-oxygen is 1.5. The two resonance structure of sulphur dioxide is

Sulphur trioxide ( $SO_3$ ): It is relatively a narrow liquid. In gaseous form it is a major pollutant that contributes to acid rain. It is a precursor to sulphuric acid and also called sulphuric anhydride. The bond order is 1.33. The vapour form of sulphur trioxide is invisible. It is transparent in liquid form. The resonance structure of sulphur trioxide is

Note:
Inhalation of sulphur dioxide and sulphur trioxide affects the respiratory tract which causes lung infection, irritation of eyes and in severe cases it causes asthma and chronic bronchitis. When these oxides come in contact with the organic materials, they are inflammable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




