
Write the decreasing order of stability of following compounds.
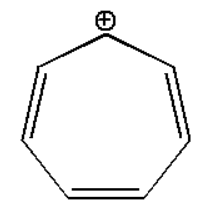
\[(i)\]
\[(ii){\text{ C}}{{\text{H}}_2} = CH - {}^ + C{H_2}\]
\[(iii){\text{ }}{{\text{C}}_6}{{\text{H}}_5} - {}^ + C{H_2}\]
\[(iv){\text{ C}}{{\text{H}}_3} - {}^ + CH - C{H_3}\]

Answer
492.9k+ views
Hint: The stability of compounds depends on the delocalization of electrons. In resonance the electrons are delocalized over the entire structure and thus its stability is most. We will analyze stability in each compound and then predict the stability of compounds.
Complete answer:
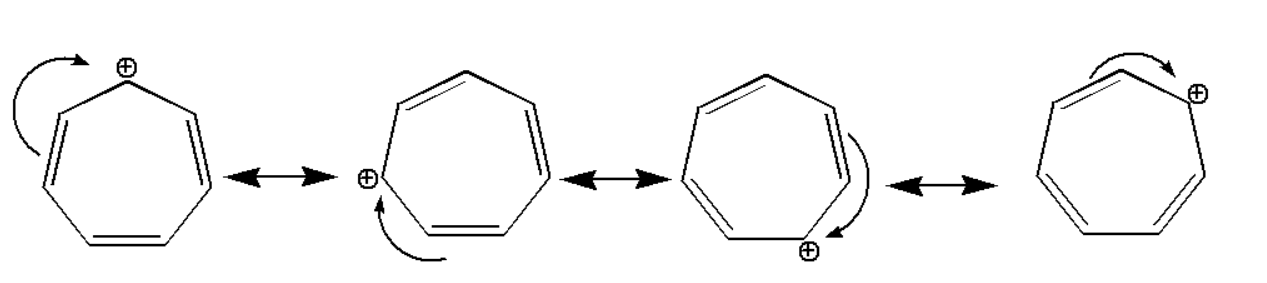
\[(i)\]:
The compound is stabilized due to resonance effect which can be shown as:
Hence we can observe that the above compound is stabilized by the resonance effect. The compound which is stabilized by resonance effect is most stabilized compound.\[(ii){\text{ C}}{{\text{H}}_2} = CH - {}^ + C{H_2}\] :
Here also the above organic compound is stabilized due to resonance effect which can be shown as:
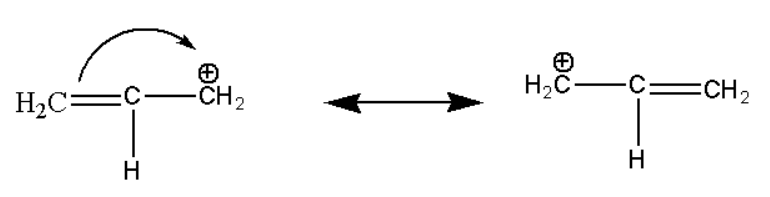
Hence the ethane molecule is stabilized by resonance effect but its stability is less as compared to stability of a ring.
\[(iii){\text{ }}{{\text{C}}_6}{{\text{H}}_5} - {}^ + C{H_2}\]
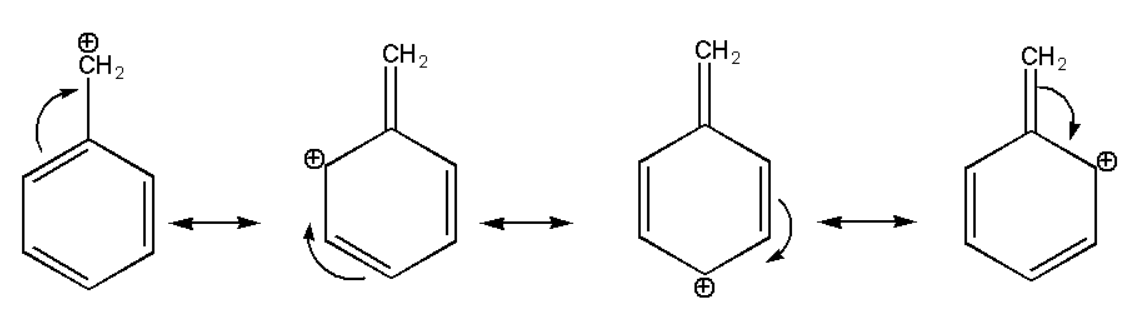
The above structure also gets stabilized due to the resonance effect of pi bonds but here the aromaticity of the benzene ring gets distorted. But its stability is also more than aliphatic compounds.
\[(iv){\text{ C}}{{\text{H}}_3} - {}^ + CH - C{H_3}\]
The above compound will be stabilized by the \[\left( { + I} \right)\] effect of the methyl group attached at both sides of carbocation. The two degree carbocation is more stable than one degree carbocation.
Since we know that the compound stabilized by the resonance effect has more stability than the compound stabilized by \[\left( { + I} \right)\] effect and hyperconjugation effect. Thus the order of stability will be as: \[(i) \succ (iii) \succ (ii) \succ (iv)\].
Note:
It must be noted that a ring stabilized by resonance effect is much more stabilized than an aliphatic compound stabilized by resonance. Also due to presence of methyl group on benzene it disturbs the aromaticity of the benzene ring. The greater the delocalization of electrons, the more stable the compound will be.
Complete answer:
\[(i)\]:
The compound is stabilized due to resonance effect which can be shown as:

Hence we can observe that the above compound is stabilized by the resonance effect. The compound which is stabilized by resonance effect is most stabilized compound.\[(ii){\text{ C}}{{\text{H}}_2} = CH - {}^ + C{H_2}\] :
Here also the above organic compound is stabilized due to resonance effect which can be shown as:

Hence the ethane molecule is stabilized by resonance effect but its stability is less as compared to stability of a ring.
\[(iii){\text{ }}{{\text{C}}_6}{{\text{H}}_5} - {}^ + C{H_2}\]

The above structure also gets stabilized due to the resonance effect of pi bonds but here the aromaticity of the benzene ring gets distorted. But its stability is also more than aliphatic compounds.
\[(iv){\text{ C}}{{\text{H}}_3} - {}^ + CH - C{H_3}\]
The above compound will be stabilized by the \[\left( { + I} \right)\] effect of the methyl group attached at both sides of carbocation. The two degree carbocation is more stable than one degree carbocation.
Since we know that the compound stabilized by the resonance effect has more stability than the compound stabilized by \[\left( { + I} \right)\] effect and hyperconjugation effect. Thus the order of stability will be as: \[(i) \succ (iii) \succ (ii) \succ (iv)\].
Note:
It must be noted that a ring stabilized by resonance effect is much more stabilized than an aliphatic compound stabilized by resonance. Also due to presence of methyl group on benzene it disturbs the aromaticity of the benzene ring. The greater the delocalization of electrons, the more stable the compound will be.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




