
Write the complete chemical reaction between magnesium and carbon dioxide and determine the nature of reaction?
Answer
510k+ views
Hint: Number of metals or non-metal combine with various reagents to form products during such reaction oxidation, reduction or even both the process can accomplish. These types of reactions are commonly known as redox reactions.
Complete answer:
Magnesium is alkali earth metal and very reactive towards different chemical reactions. When magnesium is reacting with carbon dioxide it undergoes a redox reaction to form magnesium oxide and carbon monoxide.
$2Mg + C{O_2} \to 2MgO + C$
First we understand the reaction of magnesium. initially magnesium present as neutral atom its oxidation number becomes zero.
But when it combine with oxygen to form magnesium oxide, its oxidation number changes and become-
$Mg \to MgO$
As we know oxidation number of oxygen is $\left( { - 2} \right)$
So oxidation number of $Mg$ let $\left( x \right)$ in $MgO$ is
$x + \left( { - 2} \right) = 0$
$x = 2$
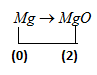
Similarly when carbon dioxide converted into nascent carbon its oxidation number changes
$C{O_2} \to C$
Oxidation number of carbon in $C{O_2}$ let $\left( x \right)$ is
$x + 2 \times \left( { - 2} \right) = 0$
$x - 4 = 0$
On solving above equation, we get the value of $\left( x \right)$
$x = 4$
Carbon atoms are present as neutral nascent atoms hence, its oxidation number is $\left( 0 \right)$.
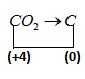
From the above calculation we see that the oxidation number of both magnesium and carbon changes during the reaction. Since, magnesium is more reactive metal than carbon it has a tendency to displace carbon from carbon dioxide to form magnesium oxide.
Hence, magnesium and carbon dioxide undergo Displacement reaction to form magnesium oxide and carbon.
$2Mg + C{O_2} \to 2MgO + C$
Note:
During chemical reaction when a less active element is displaced from its compound by a more active element is known as Displacement reaction.
In general the oxidation number of oxygen is considered as $\left( { - 2} \right)$ but it may change when oxygen forms peroxide or superoxide.
Complete answer:
Magnesium is alkali earth metal and very reactive towards different chemical reactions. When magnesium is reacting with carbon dioxide it undergoes a redox reaction to form magnesium oxide and carbon monoxide.
$2Mg + C{O_2} \to 2MgO + C$
First we understand the reaction of magnesium. initially magnesium present as neutral atom its oxidation number becomes zero.
But when it combine with oxygen to form magnesium oxide, its oxidation number changes and become-
$Mg \to MgO$
As we know oxidation number of oxygen is $\left( { - 2} \right)$
So oxidation number of $Mg$ let $\left( x \right)$ in $MgO$ is
$x + \left( { - 2} \right) = 0$
$x = 2$
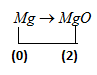
Similarly when carbon dioxide converted into nascent carbon its oxidation number changes
$C{O_2} \to C$
Oxidation number of carbon in $C{O_2}$ let $\left( x \right)$ is
$x + 2 \times \left( { - 2} \right) = 0$
$x - 4 = 0$
On solving above equation, we get the value of $\left( x \right)$
$x = 4$
Carbon atoms are present as neutral nascent atoms hence, its oxidation number is $\left( 0 \right)$.
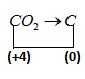
From the above calculation we see that the oxidation number of both magnesium and carbon changes during the reaction. Since, magnesium is more reactive metal than carbon it has a tendency to displace carbon from carbon dioxide to form magnesium oxide.
Hence, magnesium and carbon dioxide undergo Displacement reaction to form magnesium oxide and carbon.
$2Mg + C{O_2} \to 2MgO + C$
Note:
During chemical reaction when a less active element is displaced from its compound by a more active element is known as Displacement reaction.
In general the oxidation number of oxygen is considered as $\left( { - 2} \right)$ but it may change when oxygen forms peroxide or superoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




