
Write the chemical formula of the following:
$ A) $ Calcium sulphate
$ B) $ Potassium nitrate
$ C) $ Sodium bromide
$ D) $ Aluminium sulphate
Answer
513.9k+ views
Hint :A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, where the reactant entities are given on the left-hand side and the product entities on the right-hand side. A chemical formula is also called a molecular formula. The symbols in the molecular formula tell us about the elements and the subscript tells us how many atoms of that element are present in one molecule.
Complete Step By Step Answer:
$ - $ Write down the symbols of the elements, which combine to form a molecule of the compound, side by side. While writing the formula of a compound containing a metal and a non-metal, the symbol of the metal is written first followed by that of the non-metal.
$ - $ Write the valency of each element.
$ - $ Interchange the valencies of the elements and write as the subscript. Write them close together and ignore $ 1 $ to obtain the formula.
$ - $ The radical must be written in brackets, before the subscript is written. In case the number of polyatomic ions is one, the bracket is not required.
$ A) $ Calcium sulphate: The symbol of calcium is $ Ca $ and sulphate is $ S{O_4}^{2 - } $ . The charge on the sulphate ion is $ - 2 $ and the valency of calcium is $ 2 $ . Now by using crossing the charge method the chemical formula will be:
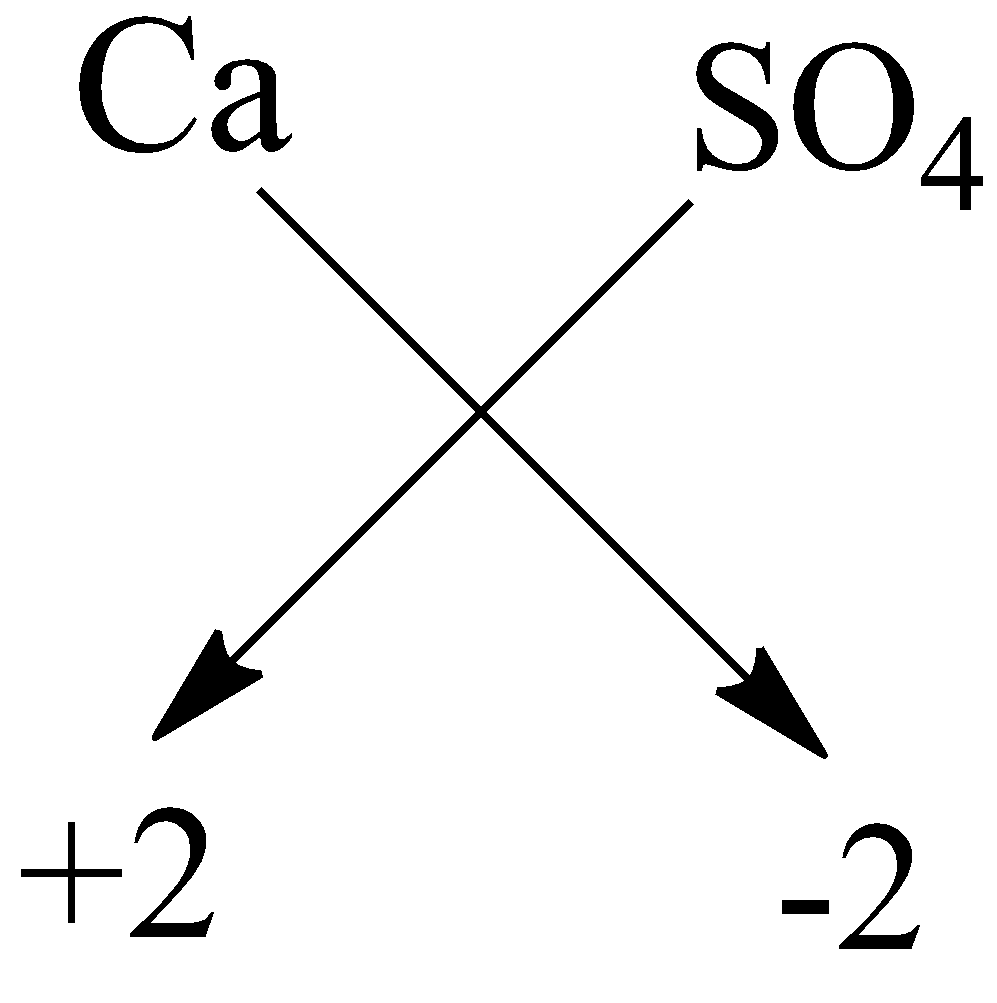
So, the formula of calcium sulphate is $ CaS{O_4} $ .
$ B) $ Potassium nitrate: The symbol of potassium is $ {K^ + } $ and nitrate is $ N{O_3}^ - $ . The charge on the potassium is $ + 1 $ and on nitrate it is $ - 1 $ . Now by using crossing the charge method the chemical formula will be:
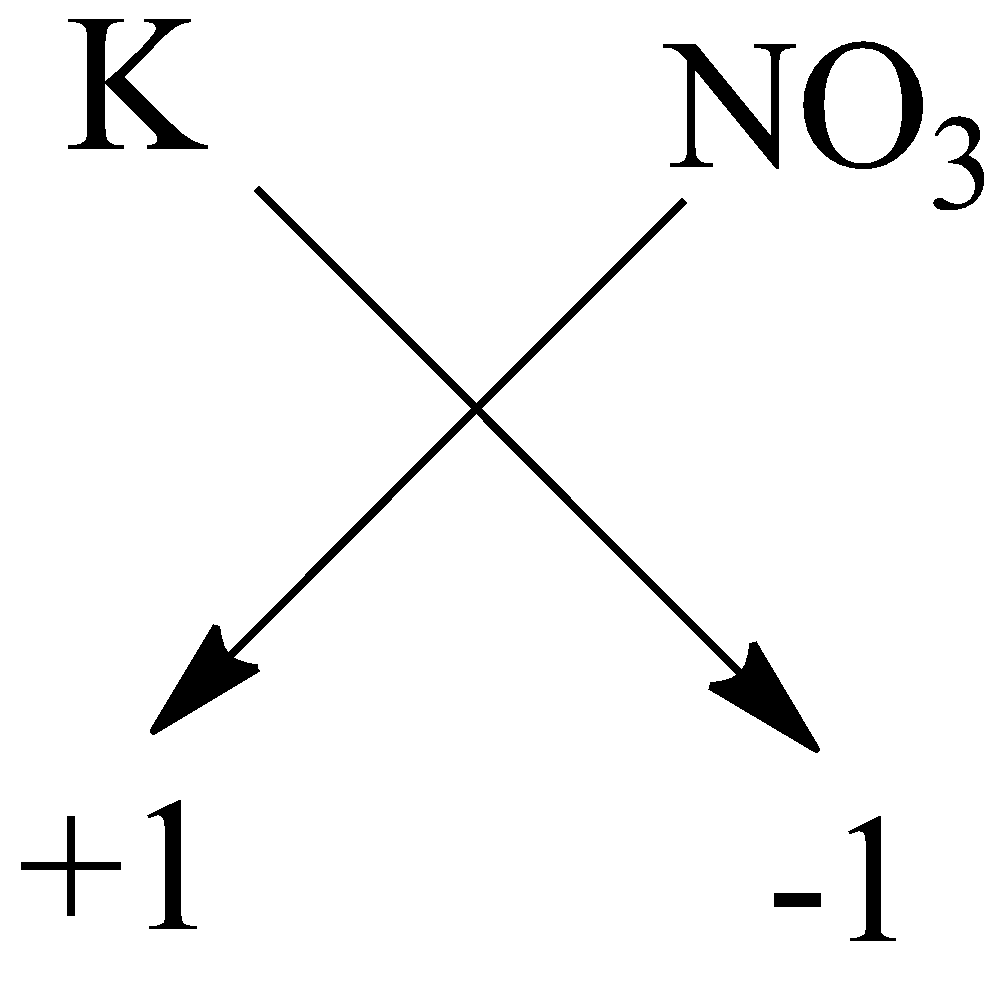
So, the chemical formula is $ KN{O_3} $ .
$ C) $ Sodium bromide: The symbol of sodium is $ Na $ and bromide is $ Br $ . The charge on the sodium is $ + 1 $ and on bromide it is $ - 1 $ . Now by using crossing the charge method the chemical formula will be:
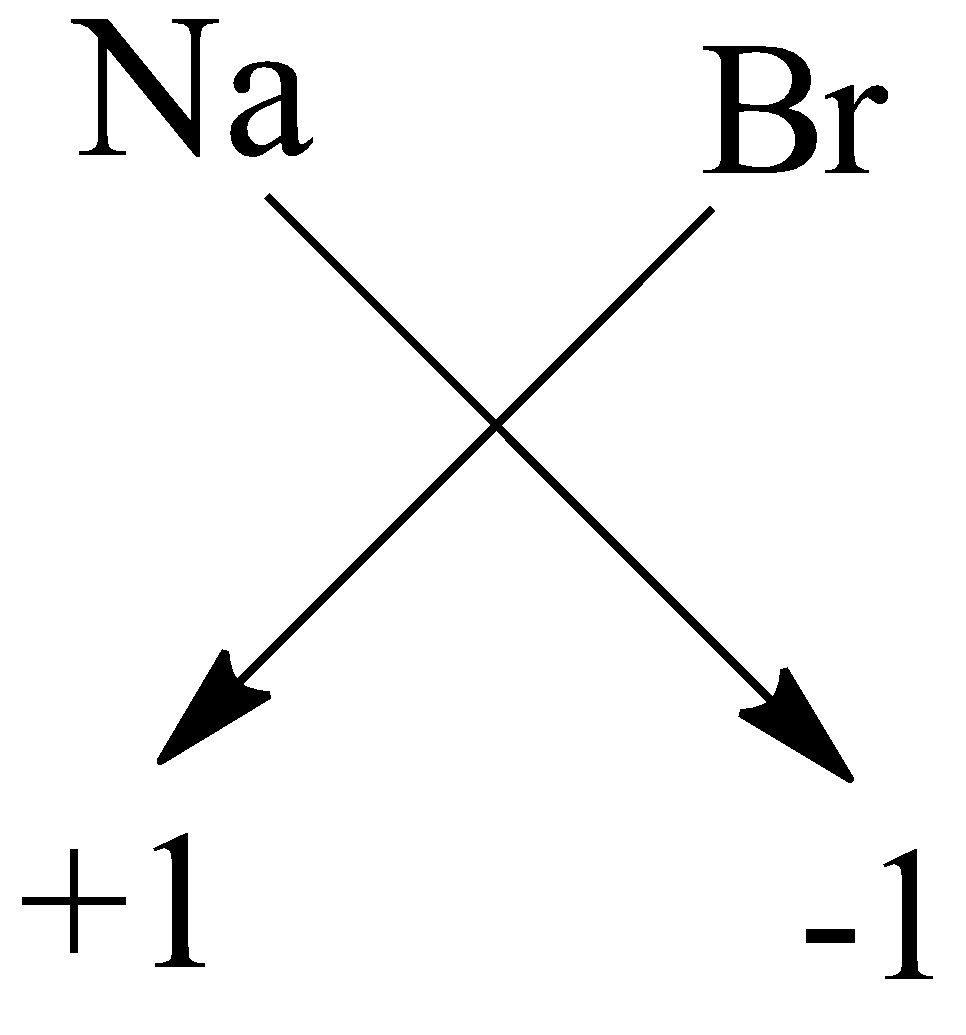
So, the chemical formula is $ NaBr $ .
$ D) $ Aluminium sulphate: the symbol of aluminium is $ Al $ and sulphate is $ S{O_4}^{2 - } $ . The valency of aluminium is $ + 3 $ and charge on sulphate is $ - 2 $ . Now by using crossing the charge method the chemical formula will be:
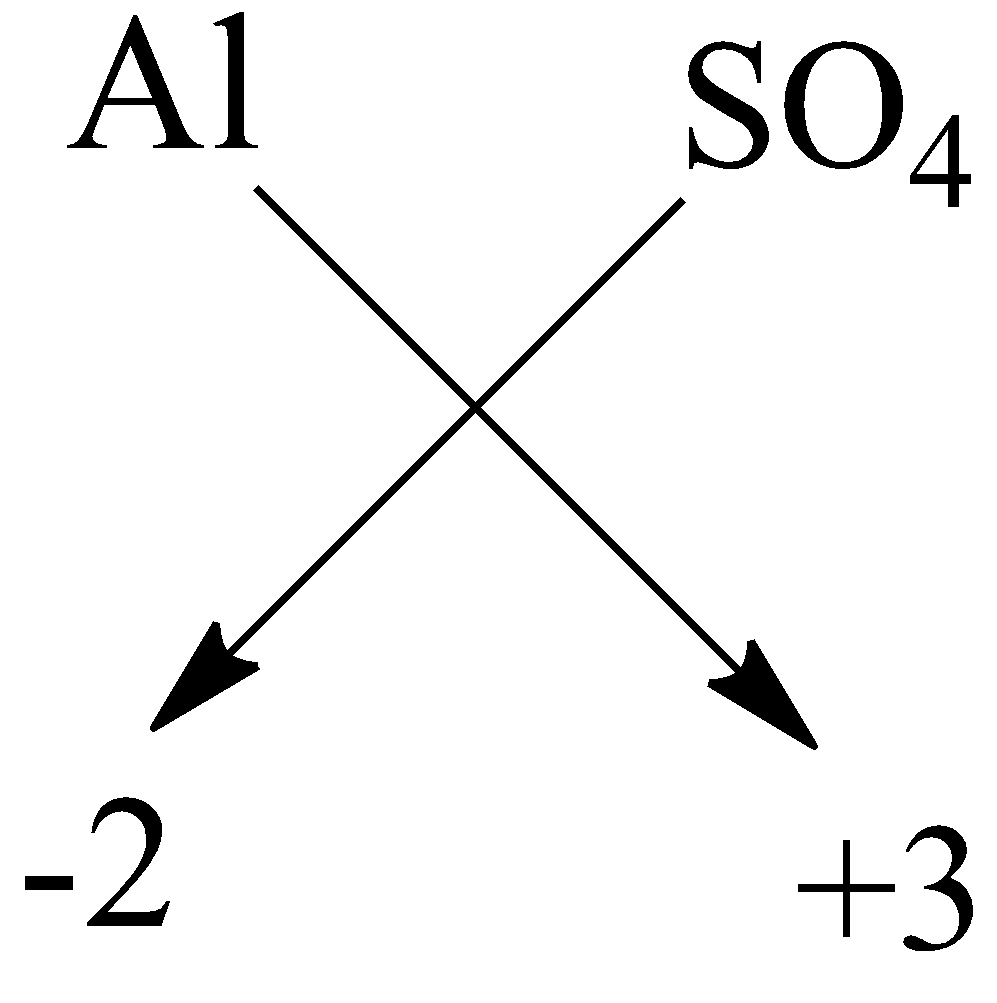
So, the chemical formula is $ A{l_2}{(S{O_4})_3} $ .
Note :
The simplest types of chemical formulae are called empirical formulas which use letters and numbers indicating the numerical proportions of atoms of each type. Molecular formulas indicate the simple numbers of each type of atom in a molecule, with no information on structure.
Complete Step By Step Answer:
$ - $ Write down the symbols of the elements, which combine to form a molecule of the compound, side by side. While writing the formula of a compound containing a metal and a non-metal, the symbol of the metal is written first followed by that of the non-metal.
$ - $ Write the valency of each element.
$ - $ Interchange the valencies of the elements and write as the subscript. Write them close together and ignore $ 1 $ to obtain the formula.
$ - $ The radical must be written in brackets, before the subscript is written. In case the number of polyatomic ions is one, the bracket is not required.
$ A) $ Calcium sulphate: The symbol of calcium is $ Ca $ and sulphate is $ S{O_4}^{2 - } $ . The charge on the sulphate ion is $ - 2 $ and the valency of calcium is $ 2 $ . Now by using crossing the charge method the chemical formula will be:

So, the formula of calcium sulphate is $ CaS{O_4} $ .
$ B) $ Potassium nitrate: The symbol of potassium is $ {K^ + } $ and nitrate is $ N{O_3}^ - $ . The charge on the potassium is $ + 1 $ and on nitrate it is $ - 1 $ . Now by using crossing the charge method the chemical formula will be:

So, the chemical formula is $ KN{O_3} $ .
$ C) $ Sodium bromide: The symbol of sodium is $ Na $ and bromide is $ Br $ . The charge on the sodium is $ + 1 $ and on bromide it is $ - 1 $ . Now by using crossing the charge method the chemical formula will be:

So, the chemical formula is $ NaBr $ .
$ D) $ Aluminium sulphate: the symbol of aluminium is $ Al $ and sulphate is $ S{O_4}^{2 - } $ . The valency of aluminium is $ + 3 $ and charge on sulphate is $ - 2 $ . Now by using crossing the charge method the chemical formula will be:

So, the chemical formula is $ A{l_2}{(S{O_4})_3} $ .
Note :
The simplest types of chemical formulae are called empirical formulas which use letters and numbers indicating the numerical proportions of atoms of each type. Molecular formulas indicate the simple numbers of each type of atom in a molecule, with no information on structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




