
Write the chemical formula for the zinc acetate and antimony trioxide.
Answer
521.4k+ views
Hint: Chemical formula: A set of chemical symbols representing the elements and their respective proportions in a chemical compound. Chemical formula of a compound can be written by using the respective valencies of an atom or formal charge of molecules comprising the compound.
Complete answer:
The chemical formula can be written according to the steps which are as follows:
1.Zinc Acetate
Step-1: Write down the symbols of element, ion or molecules used in the compound:
Symbol for Zinc atom \[ = Zn\]
Symbol for acetate ion \[ = C{H_3}CO{O^ - }\]
Step-2: Write down the valency of elements or formal charge of molecules used.
Valency of zinc atom: The valence shell of zinc i.e., \[4s\] subshell consists of two electrons i.e., it has a tendency to lose two electrons which are located in its \[4s\] subshell. Therefore, the valency of the zinc atom is \[ + 2\].
Acetate ion already exists in its ionic form, so there is no need to calculate any formal charge for it.
Step-3: The chemical formula is written according to the cross-multiplication rule.
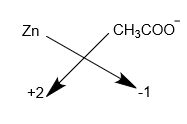
Hence, the chemical formula of the zinc acetate is \[Zn{(C{H_3}COO)_2}\]
1.Antimony trioxide
Step-1: Write down the symbols of element, ion or molecules used in the compound:
Symbol for antimony atom \[ = Sb\]
Symbol for oxygen ion \[ = O\]
Step-2: Write down the valency of elements or formal charge of molecules used.
Valency of antimony atom: The valence shell of antimony i.e., \[5p\] subshell consist of three electrons i.e., it has a tendency to lose three electrons which are located in its \[5p\] subshell. Therefore, the valency of antimony is \[ + 3\].
Valency of oxygen atom: The valence shell of oxygen i.e., \[2p\] subshell consists of four electrons i.e., it has a tendency to accept four electrons. Therefore, the valency of oxygen is \[ - 2\].
Step-3: The chemical formula is written according to the cross-multiplication rule.
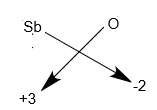
Hence, the chemical formula of the antimony trioxide is \[S{b_2}{O_3}\].
Note:
The electropositive element i.e., element having positive valency is always symbolized before the electronegative element i.e., element with negative charge. In the suffix of the symbols, charge is never mentioned, only magnitude of the valency is written.
Complete answer:
The chemical formula can be written according to the steps which are as follows:
1.Zinc Acetate
Step-1: Write down the symbols of element, ion or molecules used in the compound:
Symbol for Zinc atom \[ = Zn\]
Symbol for acetate ion \[ = C{H_3}CO{O^ - }\]
Step-2: Write down the valency of elements or formal charge of molecules used.
Valency of zinc atom: The valence shell of zinc i.e., \[4s\] subshell consists of two electrons i.e., it has a tendency to lose two electrons which are located in its \[4s\] subshell. Therefore, the valency of the zinc atom is \[ + 2\].
Acetate ion already exists in its ionic form, so there is no need to calculate any formal charge for it.
Step-3: The chemical formula is written according to the cross-multiplication rule.

Hence, the chemical formula of the zinc acetate is \[Zn{(C{H_3}COO)_2}\]
1.Antimony trioxide
Step-1: Write down the symbols of element, ion or molecules used in the compound:
Symbol for antimony atom \[ = Sb\]
Symbol for oxygen ion \[ = O\]
Step-2: Write down the valency of elements or formal charge of molecules used.
Valency of antimony atom: The valence shell of antimony i.e., \[5p\] subshell consist of three electrons i.e., it has a tendency to lose three electrons which are located in its \[5p\] subshell. Therefore, the valency of antimony is \[ + 3\].
Valency of oxygen atom: The valence shell of oxygen i.e., \[2p\] subshell consists of four electrons i.e., it has a tendency to accept four electrons. Therefore, the valency of oxygen is \[ - 2\].
Step-3: The chemical formula is written according to the cross-multiplication rule.

Hence, the chemical formula of the antimony trioxide is \[S{b_2}{O_3}\].
Note:
The electropositive element i.e., element having positive valency is always symbolized before the electronegative element i.e., element with negative charge. In the suffix of the symbols, charge is never mentioned, only magnitude of the valency is written.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




