
How do you write the chemical formula for:
1. Gold (III) acetate.
2. Tungsten (VI) bromate.
Answer
546.6k+ views
Hint: Chemical formula is basically a method for the presentation of information about the chemical compositions and proportions of different atoms that constitute a particular molecule or chemical compound. We basically write the chemical formula of a compound by crisscrossing the valency of elements involved.
Complete step-by-step answer:
The first thing which we need to keep in mind while writing a chemical formula is to know about the symbolic representation of the elements or group of elements involved in the compounds.
The first compound is gold (III) acetate. We should remember that gold is represented by the chemical symbol “Ag” and acetate ion is represented by $C{{H}_{3}}CO{{O}^{-}}$.
The second compound is tungsten (VI) bromate. Tungsten is represented by “W” whereas Bromate is represented by $BrO_{3}^{-}$.
Next step is to identify cation and anion. Always remember that in a given compound the first element is always cation and the another is anion. Therefore, in gold (III) acetate gold is the cation whereas acetate is the anion. And in tungsten (VI) bromate, tungsten is the cation whereas bromate is the anion.
Also keep in mind that roman numeral notation in parentheses is just the oxidation state of the cation. Therefore, gold has $+3$ charge in it and for the acetate we know that it has a charge of $-1$
Similarly, for the tungsten the charge is $+6$ and bromate we know has a charge of $-1$
For the sake of simplicity, we take the charges on the cations and anions as their valency. So, we can say that valency of gold and acetate is $+3$ and $-1$ respectively. Similarly, valency of tungsten and bromate is $+6$ and $-1$ in this question.
Now we need to just crisscross the valency of the elements as shown below:

Therefore, the chemical formula for gold (III) acetate is $Au{{\left( C{{H}_{3}}COO \right)}_{3}}$
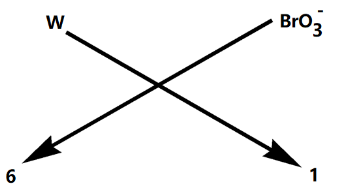
Therefore, the chemical formula for tungsten (VI) bromate is $W{{\left( Br{{O}_{3}} \right)}_{6}}$
Note: Students should that always while writing the chemical formula only consider the numerical part of the charge and never consider the sign. Here, in this question also we only considered the numerical value 1 and didn’t consider the negative charge. Always remember that you have to cross multiply the charges and not self-multiply them.
Complete step-by-step answer:
The first thing which we need to keep in mind while writing a chemical formula is to know about the symbolic representation of the elements or group of elements involved in the compounds.
The first compound is gold (III) acetate. We should remember that gold is represented by the chemical symbol “Ag” and acetate ion is represented by $C{{H}_{3}}CO{{O}^{-}}$.
The second compound is tungsten (VI) bromate. Tungsten is represented by “W” whereas Bromate is represented by $BrO_{3}^{-}$.
Next step is to identify cation and anion. Always remember that in a given compound the first element is always cation and the another is anion. Therefore, in gold (III) acetate gold is the cation whereas acetate is the anion. And in tungsten (VI) bromate, tungsten is the cation whereas bromate is the anion.
Also keep in mind that roman numeral notation in parentheses is just the oxidation state of the cation. Therefore, gold has $+3$ charge in it and for the acetate we know that it has a charge of $-1$
Similarly, for the tungsten the charge is $+6$ and bromate we know has a charge of $-1$
For the sake of simplicity, we take the charges on the cations and anions as their valency. So, we can say that valency of gold and acetate is $+3$ and $-1$ respectively. Similarly, valency of tungsten and bromate is $+6$ and $-1$ in this question.
Now we need to just crisscross the valency of the elements as shown below:

Therefore, the chemical formula for gold (III) acetate is $Au{{\left( C{{H}_{3}}COO \right)}_{3}}$

Therefore, the chemical formula for tungsten (VI) bromate is $W{{\left( Br{{O}_{3}} \right)}_{6}}$
Note: Students should that always while writing the chemical formula only consider the numerical part of the charge and never consider the sign. Here, in this question also we only considered the numerical value 1 and didn’t consider the negative charge. Always remember that you have to cross multiply the charges and not self-multiply them.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




