
Write T for the correct statement and F for the wrong statement in a sequence.
a) Oil can easily mix with water.
b) Sugar and salt do not dissolve in water.
c) Salt is made from seawater.
d) Sugar and salt do not dissolve in hot water.
(A) FFTF
(B) FFFF
(C) TFTF
(D) FFTT
Answer
530.7k+ views
Hint: To solve this question, we need to verify each of the statements given above regarding the solubility of compounds. The ability of a substance (or the solute) to dissolve in a liquid (or a solvent) to form a solution is known as solubility. Aqueous solutions are made when a solute is dissolved in water.
Complete answer:
a) The statement that oils can easily mix with water is false.
This is because the bonds that hold together water (liquid) are hydrogen bonds. Since there are no polar parts in oils, they need to break the hydrogen bonds in water to dissolve in it.
But since the hydrogen bonds are strong, the water molecules are more attracted to each other instead of the oil molecules, and hence oils and water do not mix easily.
b) The statement that sugar and salt do not dissolve in water is false.
We know that salts are ionic chemical compounds that contain cations (positively charged ions) and anions (negatively charged ions) such that the molecule is electrically neutral.
Since these are extremely polar substances, they are easily soluble in polar solvents like water, as they dissociate into their constituent cationic and anionic components.
Now, sugar is water-soluble carbohydrates that have a sweet taste and are generally used in food.
For example, the most common sugar is a disaccharide carbohydrate named sucrose or table sugar and it contains glucose and fructose. Its structure is as follows
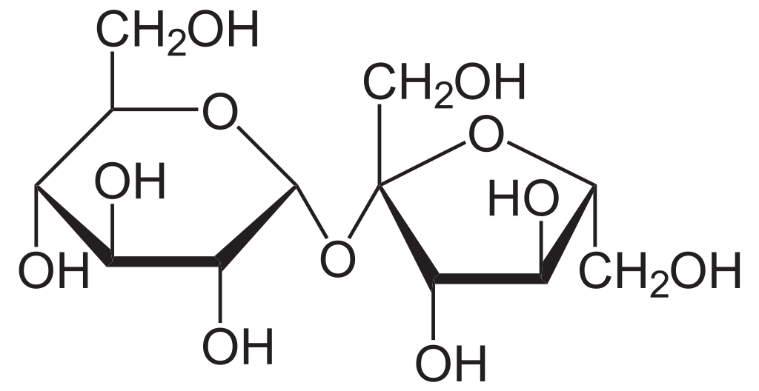
We can see that the molecule has bonds between the hydrogen atom and the oxygen atom (like water), and the charge difference between these atoms makes it a polar molecule. Hence it dissolves in polar solvents like water.
- The statement that salt is made from seawater is true.
The ions, sodium $N{{a}^{+}}$ , and chlorine $C{{l}^{-}}$ make up about 90% of the dissolved ions present in seawater. Hence the evaporation of seawater produces salts and most table salt NaCl.
- The statement that sugar and salt do not dissolve in hot water is false.
We have already established that salts and sugars are soluble in water. Now, since with an increase in temperature, solubility increases, hence sugars and salts are soluble in hot water.
So, the correct answer is option (A) FFTF.
Note:
It should be noted that
- Oil can be dissolved in water using an emulsifier. The hydrophobic end of the emulsifier surrounds the oil molecules and the hydrophilic end is exposed to water, hence making the components miscible.
- The solubility of a solute decreases with a decrease in temperature.
Complete answer:
a) The statement that oils can easily mix with water is false.
This is because the bonds that hold together water (liquid) are hydrogen bonds. Since there are no polar parts in oils, they need to break the hydrogen bonds in water to dissolve in it.
But since the hydrogen bonds are strong, the water molecules are more attracted to each other instead of the oil molecules, and hence oils and water do not mix easily.
b) The statement that sugar and salt do not dissolve in water is false.
We know that salts are ionic chemical compounds that contain cations (positively charged ions) and anions (negatively charged ions) such that the molecule is electrically neutral.
Since these are extremely polar substances, they are easily soluble in polar solvents like water, as they dissociate into their constituent cationic and anionic components.
Now, sugar is water-soluble carbohydrates that have a sweet taste and are generally used in food.
For example, the most common sugar is a disaccharide carbohydrate named sucrose or table sugar and it contains glucose and fructose. Its structure is as follows

We can see that the molecule has bonds between the hydrogen atom and the oxygen atom (like water), and the charge difference between these atoms makes it a polar molecule. Hence it dissolves in polar solvents like water.
- The statement that salt is made from seawater is true.
The ions, sodium $N{{a}^{+}}$ , and chlorine $C{{l}^{-}}$ make up about 90% of the dissolved ions present in seawater. Hence the evaporation of seawater produces salts and most table salt NaCl.
- The statement that sugar and salt do not dissolve in hot water is false.
We have already established that salts and sugars are soluble in water. Now, since with an increase in temperature, solubility increases, hence sugars and salts are soluble in hot water.
So, the correct answer is option (A) FFTF.
Note:
It should be noted that
- Oil can be dissolved in water using an emulsifier. The hydrophobic end of the emulsifier surrounds the oil molecules and the hydrophilic end is exposed to water, hence making the components miscible.
- The solubility of a solute decreases with a decrease in temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




