
Write short note on buckyball
Answer
564.9k+ views
Hint: Buckyballs are also known as fullerenes or buckminsterfullerene. Buckyballs were one of the first nanoparticles invented. These nanoparticles were discovered in 1985 by a group of three researchers namely Richard Smalley, Harry Kroto, and Robert Curl, working at Rice University.
Complete step by step answer:
Buckyballs are carbon atoms connected to three other carbon atoms via covalent bonds. However, the carbon atoms are connected to each other in the pattern similar to hexagons and pentagons (cage-like fused-ring structure) as visible on a soccer ball, thus, giving a buckyball the spherical structure. The most general form of buckyball comprises 60 carbon atoms and is therefore called as $C_60$ (as shown in the figure below). The other sizes of buckyballs vary from those containing 20 carbon atoms to those containing even more than 100 carbon atoms.
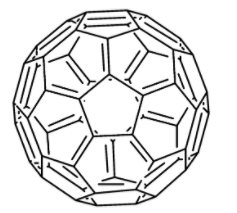
Buckyball is a black solid having the ability to dissolve in hydrocarbon solvents to generate a violet solution. The molecule is usually present in small quantities in soot. The molecule has also been found in deep space. Moreover, in April 2019, scientists carrying out research with the Hubble Space Telescope, have reported the confirmed occurrence of the huge and complex ionized molecules of buckyballs ($C_60$) in the interstellar medium spaces between the stars.
Note: The covalent bonds between carbon atoms make the buckyballs very strong, and the carbon atoms easily form covalent bonds with other atoms. Buckyballs are also utilized in composites to strengthen the material. Moreover, buckyballs possess the electrical property of being very good electron acceptors, thereby indicating that they accept loose electrons from other materials. This property is useful in increasing the efficacy of solar cells in transforming sunlight into electricity.
Complete step by step answer:
Buckyballs are carbon atoms connected to three other carbon atoms via covalent bonds. However, the carbon atoms are connected to each other in the pattern similar to hexagons and pentagons (cage-like fused-ring structure) as visible on a soccer ball, thus, giving a buckyball the spherical structure. The most general form of buckyball comprises 60 carbon atoms and is therefore called as $C_60$ (as shown in the figure below). The other sizes of buckyballs vary from those containing 20 carbon atoms to those containing even more than 100 carbon atoms.

Buckyball is a black solid having the ability to dissolve in hydrocarbon solvents to generate a violet solution. The molecule is usually present in small quantities in soot. The molecule has also been found in deep space. Moreover, in April 2019, scientists carrying out research with the Hubble Space Telescope, have reported the confirmed occurrence of the huge and complex ionized molecules of buckyballs ($C_60$) in the interstellar medium spaces between the stars.
Note: The covalent bonds between carbon atoms make the buckyballs very strong, and the carbon atoms easily form covalent bonds with other atoms. Buckyballs are also utilized in composites to strengthen the material. Moreover, buckyballs possess the electrical property of being very good electron acceptors, thereby indicating that they accept loose electrons from other materials. This property is useful in increasing the efficacy of solar cells in transforming sunlight into electricity.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




