
Write one difference between transition elements and p-block elements with reference to variability of oxidation states.
Answer
577.8k+ views
Hint: The d-block elements vary from the p-block elements with respect to the location where the last electron enters the atoms of these blocks. This causes variations in the oxidation states of elements of both the blocks.
Complete step by step solution:
-The periodic table is divided in 4 different blocks namely s-block, p-block, d-block and f-block. They show different variations with respect to the change in the oxidation states of the atoms as the electrons present in the valence shell are different in different blocks.
-The different blocks are classified on the basis of the location of the last valence electron entering the shell of the atom. In s-block elements, the valence electrons enter the s-subshell and in p-block, the valence electrons enter the p-subshell. In d-block, the valence electron enters the d-subshell due to the Aufbau’s principle.
-The configuration of p-block elements can be shown as $n{{s}^{2}}n{{p}^{1-6}}$ while that of d-block can be shown as $\left( n-1 \right){{d}^{1-10}}n{{s}^{0,1,2}}$ . This is the main reason for the difference in the oxidation states of both the p-block and d-block elements.
-The oxidation state of d-block elements varies by 1 unit only while that of p-block elements varies by 2 units. This is because the electrons are excited from the s-subshell in p-block elements creating the number of unpaired electrons as 2. In d-block elements, electrons are lost and excited from the d-subshell and not from s-subshell in their second oxidation states.
-If we take the example of Mn and S, we see that S has 6 electrons in the valence shell and its different covalencies can be formed as
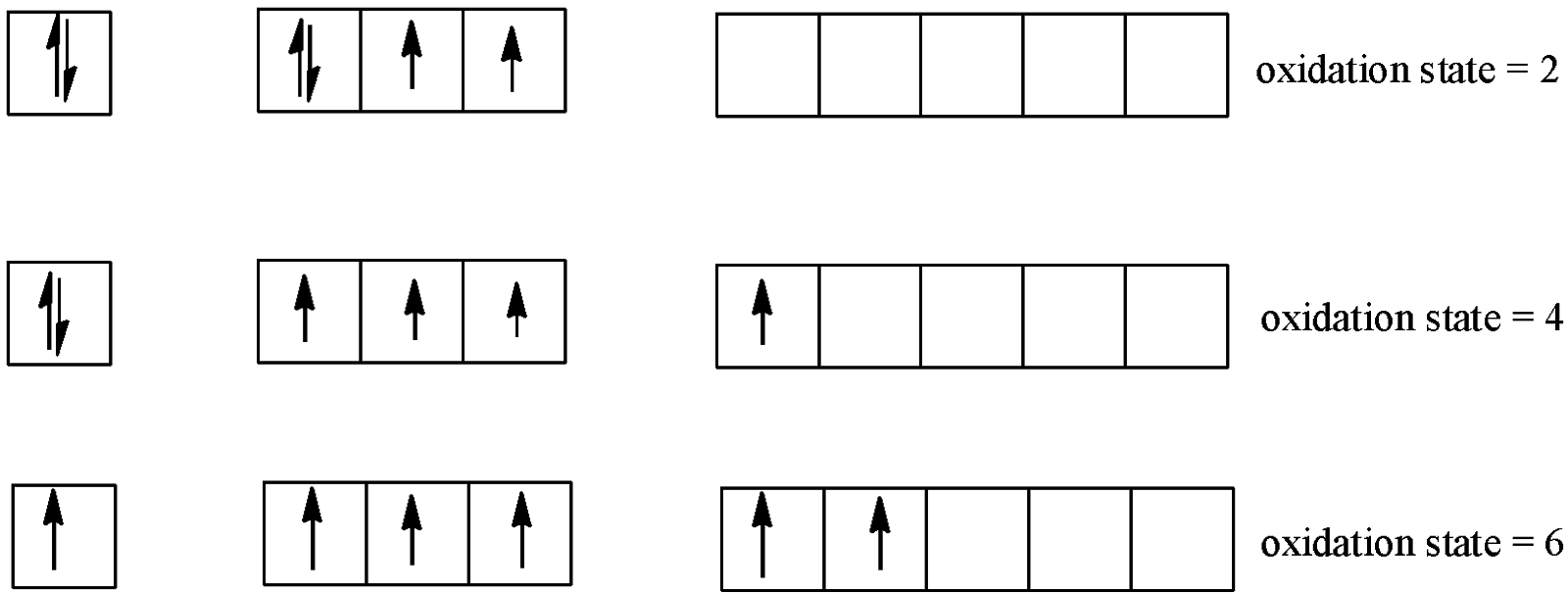
- Mn atoms form a cation with charge +2 if 2 electrons are removed from it. The outermost shell is 4s. So, when 2 electrons are removed, the configuration will become $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$. The other electron is now removed from the d-subshell itself and so the change in the oxidation state is 1 only and not 2 unlike S.
Thus we see the difference between the transition elements and p-block elements with reference to variability of oxidation states.
Note: It is due to the different behavior of the variation in the oxidation state of the p-block and the d-block elements that they show different colours. In p-block elements, only the halogens are coloured while most of the d-block elements show colour in their compounds.
Complete step by step solution:
-The periodic table is divided in 4 different blocks namely s-block, p-block, d-block and f-block. They show different variations with respect to the change in the oxidation states of the atoms as the electrons present in the valence shell are different in different blocks.
-The different blocks are classified on the basis of the location of the last valence electron entering the shell of the atom. In s-block elements, the valence electrons enter the s-subshell and in p-block, the valence electrons enter the p-subshell. In d-block, the valence electron enters the d-subshell due to the Aufbau’s principle.
-The configuration of p-block elements can be shown as $n{{s}^{2}}n{{p}^{1-6}}$ while that of d-block can be shown as $\left( n-1 \right){{d}^{1-10}}n{{s}^{0,1,2}}$ . This is the main reason for the difference in the oxidation states of both the p-block and d-block elements.
-The oxidation state of d-block elements varies by 1 unit only while that of p-block elements varies by 2 units. This is because the electrons are excited from the s-subshell in p-block elements creating the number of unpaired electrons as 2. In d-block elements, electrons are lost and excited from the d-subshell and not from s-subshell in their second oxidation states.
-If we take the example of Mn and S, we see that S has 6 electrons in the valence shell and its different covalencies can be formed as

- Mn atoms form a cation with charge +2 if 2 electrons are removed from it. The outermost shell is 4s. So, when 2 electrons are removed, the configuration will become $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}$. The other electron is now removed from the d-subshell itself and so the change in the oxidation state is 1 only and not 2 unlike S.
Thus we see the difference between the transition elements and p-block elements with reference to variability of oxidation states.
Note: It is due to the different behavior of the variation in the oxidation state of the p-block and the d-block elements that they show different colours. In p-block elements, only the halogens are coloured while most of the d-block elements show colour in their compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




