
Write IUPAC names of all the isomers of ${C_5}{H_{10}}$.
Answer
577.2k+ views
Hint:Isomers are molecules which have the same molecular formula that is the same number of atoms of each element but different arrangements of atoms in space or have different molecular structure. Isomers also have different physical and chemical properties.
Complete step by step answer:
-The chemical formula is ${C_5}{H_{10}}$.
-This chemical formula is similar to the chemical formula of alkenes and cycloalkanes. This means the isomers of ${C_5}{H_{10}}$ are either alkanes or cycloalkanes.
-The alkenes isomer of ${C_5}{H_{10}}$ are shown in the figure below:
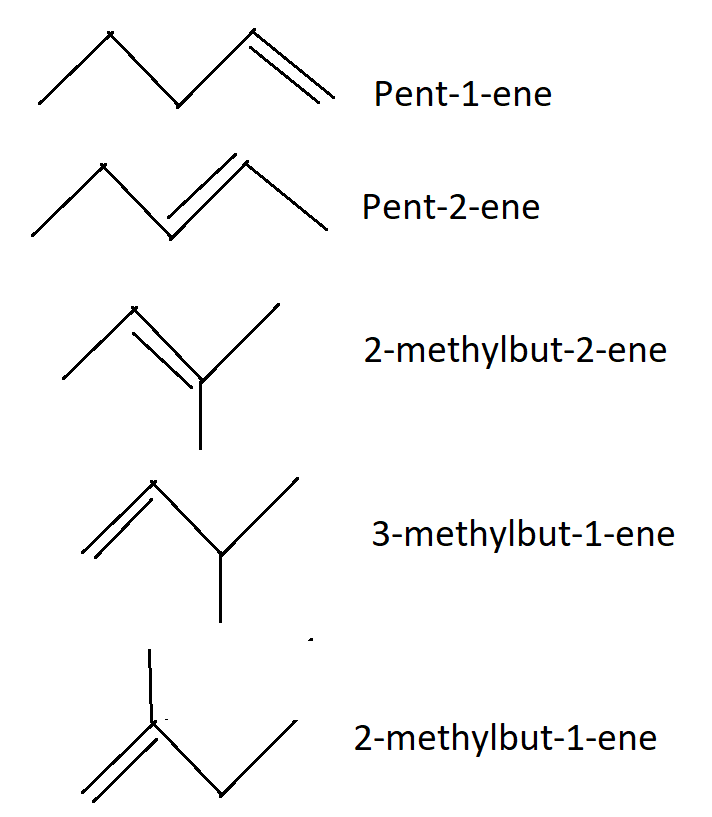
-In the above diagram, we can see that five alkenes have the same molecular formula and have a different structure. Also, all the isomers are formed by changing the position of some elements or atoms.
-Similarly, there are also some cycloalkanes with the molecular formula ${C_5}{H_{10}}$. And these cycloalkanes are shown in the figure below:
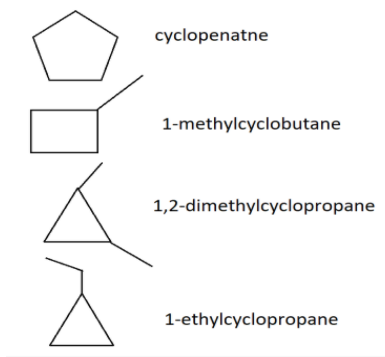
-There are four cycloalkanes which are isomers of ${C_5}{H_{10}}$.
-Then there are nine isomers of ${C_5}{H_{10}}$, and there IUPAC names are:
1.pent-1-ene
2.pent-2-ene
3.2-methylbut-2-ene
4.3-methylbut-1-ene
5.2-methylbut-2-ene
6.cyclopentane
7.1-methylcyclobutane
8.1,2-dimethylcyclopropane
9.1-ethylcyclopropane
Note:
Isomers have the same chemical formula but have different physical and chemical properties. Melting and boiling point of two isomers are different and there is a large difference. Isomers also show different chemical behavior to different chemicals. Also, functional groups may be different in isomers.
Complete step by step answer:
-The chemical formula is ${C_5}{H_{10}}$.
-This chemical formula is similar to the chemical formula of alkenes and cycloalkanes. This means the isomers of ${C_5}{H_{10}}$ are either alkanes or cycloalkanes.
-The alkenes isomer of ${C_5}{H_{10}}$ are shown in the figure below:

-In the above diagram, we can see that five alkenes have the same molecular formula and have a different structure. Also, all the isomers are formed by changing the position of some elements or atoms.
-Similarly, there are also some cycloalkanes with the molecular formula ${C_5}{H_{10}}$. And these cycloalkanes are shown in the figure below:

-There are four cycloalkanes which are isomers of ${C_5}{H_{10}}$.
-Then there are nine isomers of ${C_5}{H_{10}}$, and there IUPAC names are:
1.pent-1-ene
2.pent-2-ene
3.2-methylbut-2-ene
4.3-methylbut-1-ene
5.2-methylbut-2-ene
6.cyclopentane
7.1-methylcyclobutane
8.1,2-dimethylcyclopropane
9.1-ethylcyclopropane
Note:
Isomers have the same chemical formula but have different physical and chemical properties. Melting and boiling point of two isomers are different and there is a large difference. Isomers also show different chemical behavior to different chemicals. Also, functional groups may be different in isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




