
Write Fischer projection formula of erythrose sugar.
Answer
578.1k+ views
Hint: Basically, a Fischer projection formula is used to depict a stereo formula in two dimensions without destroying the stereo chemical information i.e. the absolute configuration at chiral centers. To solve this question, we need to know the formula of erythrose sugar and its properties.
Complete step by step answer:
So, first of all let’s discuss Erythrose. Erythrose was first isolated in the year 1849 by the French pharmacist Louis Feud Joseph Garrote. It is a tetrode saccharide with the chemical formula ${C_4}{H_8}{O_4}$. It basically consists of one aldehyde group and is a part of the aldose family.
Moreover, there are different types of arrangements of atoms. These arrangements are better known as conformational isomers. Further, they are of two types i.e. eclipse conformation and staggered conformation. In eclipse conformation, hydrogen atoms are attached to two carbons and are nearest to each other. Moreover, in case of staggered conformation, hydrogen atoms attached to two carbons are as far as possible with respect to each other. This type of conformation is relatively more stable than eclipsed conformation.
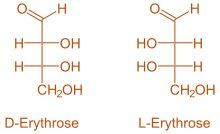
Now, in Fischer projection formula, the longest chain of carbon atoms is shown by vertical line and the branches and groups attached to the chain are shown by a horizontal line. So, the structures of D-Erythrose and L-Erythrose are as shown:
Note: Oxidative bacteria can be made to use erythrose as its sole energy source. Moreover, Erythrose-4-phosphate is an intermediate in the pentose phosphate pathway and the Calvin cycle.
Complete step by step answer:
So, first of all let’s discuss Erythrose. Erythrose was first isolated in the year 1849 by the French pharmacist Louis Feud Joseph Garrote. It is a tetrode saccharide with the chemical formula ${C_4}{H_8}{O_4}$. It basically consists of one aldehyde group and is a part of the aldose family.
Moreover, there are different types of arrangements of atoms. These arrangements are better known as conformational isomers. Further, they are of two types i.e. eclipse conformation and staggered conformation. In eclipse conformation, hydrogen atoms are attached to two carbons and are nearest to each other. Moreover, in case of staggered conformation, hydrogen atoms attached to two carbons are as far as possible with respect to each other. This type of conformation is relatively more stable than eclipsed conformation.
Now, in Fischer projection formula, the longest chain of carbon atoms is shown by vertical line and the branches and groups attached to the chain are shown by a horizontal line. So, the structures of D-Erythrose and L-Erythrose are as shown:

Note: Oxidative bacteria can be made to use erythrose as its sole energy source. Moreover, Erythrose-4-phosphate is an intermediate in the pentose phosphate pathway and the Calvin cycle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




