
Write down the structures of stereoisomers formed when cis-2-butene is treated with bromine.
Answer
605.4k+ views
Hint: In order to draw the stereoisomers at first we need to know the formula of the parent molecule. From the molecular structure of the parent molecule we can derive the stereoisomers. In the answer which is mentioned below we have been classifying the various ways in which the ions participating in the chemical reactions can be rearranged, and the final products are also mentioned.
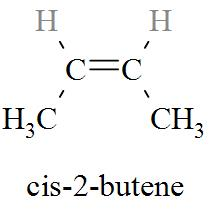
Complete step by step solution: The molecular formula of cis-2-butene is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$. From the molecular formula of cis-2-butene, we can derive the molecular structure of cis-2-butene
When cis-2-butene is treated with bromine, Bromonium ion is formed. The chemical reaction is shown in details in the following diagram.
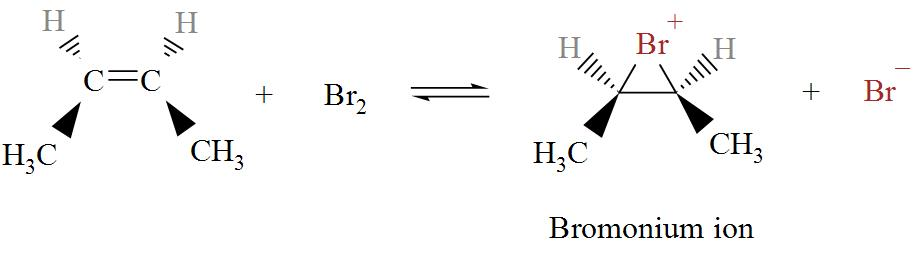
In the following diagram we can see bromine ion attacking the Bromonium ions in two possible ways.
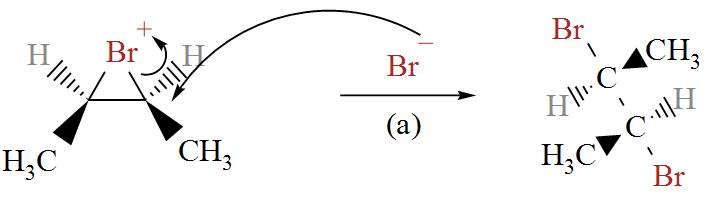
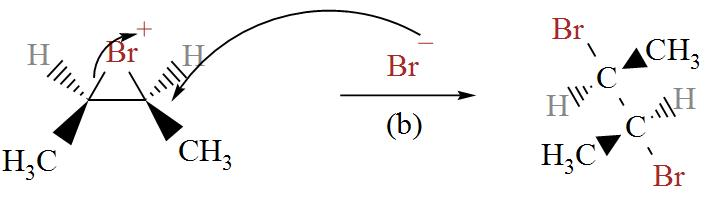
From here, we get two stereoisomers of cis-2-butene, (2R, 3R) and (2S, 3S).

These are the end-products when cis-2-butene reacts with Bromine.
Note: In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of the atoms in space.
Structural isomers are basically classified as those compounds which differ in their connectivity between the constituent atoms that make up the component. However the stereoisomers should not be confused with them because they have the exact same molecular formula. The only thing that differs is the arrangement of the atoms in the three-dimensional space.
Complete step by step solution: The molecular formula of cis-2-butene is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$. From the molecular formula of cis-2-butene, we can derive the molecular structure of cis-2-butene

When cis-2-butene is treated with bromine, Bromonium ion is formed. The chemical reaction is shown in details in the following diagram.
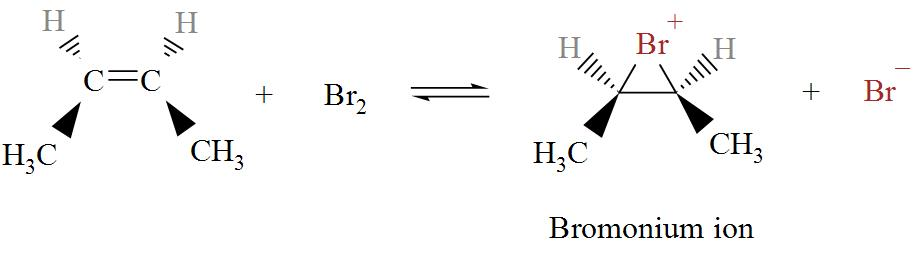
In the following diagram we can see bromine ion attacking the Bromonium ions in two possible ways.


From here, we get two stereoisomers of cis-2-butene, (2R, 3R) and (2S, 3S).

These are the end-products when cis-2-butene reacts with Bromine.
Note: In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of the atoms in space.
Structural isomers are basically classified as those compounds which differ in their connectivity between the constituent atoms that make up the component. However the stereoisomers should not be confused with them because they have the exact same molecular formula. The only thing that differs is the arrangement of the atoms in the three-dimensional space.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




