
Write down the formula of the compound formed between C and D.
A. $\text{CD}$
B. $\text{C}{{\text{D}}_{2}}$
C. $\text{C}{{\text{D}}_{4}}$
D. $\text{C}{{\text{D}}_{3}}$
Answer
585.9k+ views
Hint: The question is all about bond formation. The number of bonds formed by the element with another element depends on the valency of that element (especially elements which don’t have d-orbitals).
Complete answer:
Before dealing with the question, let us define what is valency, valency is the count of the maximum number of atoms that an element can lose or gain to achieve inert gas configuration (noble gas configuration). Carbon belongs to period 2 and have configuration as $2,4\text{ or 1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{2}}$. So, the valency of carbon will be 4 as there are 4 valence electrons in it.
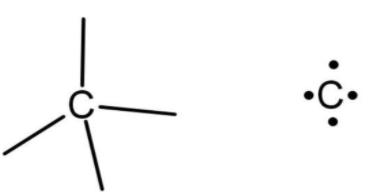
Now, talking about deuterium, it is an isotope of hydrogen with the number of protons as 1 and the number of electrons as 1. While forming bonds with other elements deuterium resembles hydrogen. It has the same valency as hydrogen which is 1.
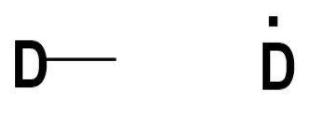
So, the four valencies or vacancies on carbon atoms can be filled by four deuterium atoms. One electron from deuterium and one electron from the carbon atom will combine to form a bond. There are four valence electrons on carbon atoms. Thus, the structure formed will be
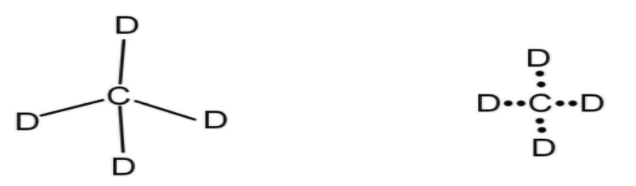
The molecular formula of this structure will be $\dfrac{\text{C}}{1}\searrow \dfrac{\text{D}}{4}\text{ and }\dfrac{\text{C}}{1}\nearrow \dfrac{\text{D}}{4}$, so, the chemical formula will be $\text{C}{{\text{D}}_{4}}$.
The correct answer to this question is the formula of compound formed between carbon and deuterium is$\text{C}{{\text{D}}_{4}}$, which is option ‘c’.
Note:
The compound that can also be formed by the reaction of and is ${{\text{C}}_{2}}{{\text{D}}_{2}}$ just like ${{\text{C}}_{2}}{{\text{H}}_{2}}$ (acetylene). The structure of ${{\text{C}}_{2}}{{\text{D}}_{2}}$ is

Complete answer:
Before dealing with the question, let us define what is valency, valency is the count of the maximum number of atoms that an element can lose or gain to achieve inert gas configuration (noble gas configuration). Carbon belongs to period 2 and have configuration as $2,4\text{ or 1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{2}}$. So, the valency of carbon will be 4 as there are 4 valence electrons in it.

Now, talking about deuterium, it is an isotope of hydrogen with the number of protons as 1 and the number of electrons as 1. While forming bonds with other elements deuterium resembles hydrogen. It has the same valency as hydrogen which is 1.
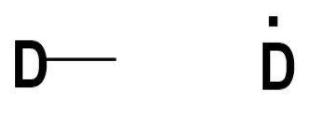
So, the four valencies or vacancies on carbon atoms can be filled by four deuterium atoms. One electron from deuterium and one electron from the carbon atom will combine to form a bond. There are four valence electrons on carbon atoms. Thus, the structure formed will be
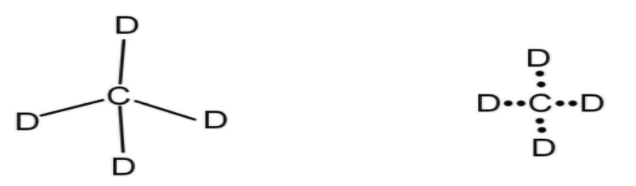
The molecular formula of this structure will be $\dfrac{\text{C}}{1}\searrow \dfrac{\text{D}}{4}\text{ and }\dfrac{\text{C}}{1}\nearrow \dfrac{\text{D}}{4}$, so, the chemical formula will be $\text{C}{{\text{D}}_{4}}$.
The correct answer to this question is the formula of compound formed between carbon and deuterium is$\text{C}{{\text{D}}_{4}}$, which is option ‘c’.
Note:
The compound that can also be formed by the reaction of and is ${{\text{C}}_{2}}{{\text{D}}_{2}}$ just like ${{\text{C}}_{2}}{{\text{H}}_{2}}$ (acetylene). The structure of ${{\text{C}}_{2}}{{\text{D}}_{2}}$ is

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




