
Write down the formula of sodium sulphate using the criss cross method.
Answer
533.1k+ views
Hint: There is an old method to write the chemical formula of an ionic compound very easily and the method name is criss cross method. We have to follow a few steps to write the chemical formula of the compound by using the criss cross method.
Complete answer:
- In the question it is asked to write the chemical formula of the sodium sulphate by using the criss cross method.
- Few steps we have to follow to write the chemical formula of the sodium sulphate and they are as follows.
1) We have to identify the cation and anion of the chemical and have to write the cation symbol first and later anion method like as follows.
- In the chemical compound we know that sodium is cation and sulphate is anion.
\[\underset{Cation}{\mathop{\text{Na }}}\,\text{ }\underset{Anion}{\mathop{\text{S}{{\text{O}}_{\text{4}}}}}\,\]
2) We have to write the charge of each ion at their bottom like this:
\[\underset{1\text{ }}{\mathop{\underset{Cation}{\mathop{\text{Na }}}\,\text{ }}}\,\text{ }\underset{\text{2}}{\mathop{\text{ }\underset{Anion}{\mathop{\text{S}{{\text{O}}_{\text{4}}}}}\,}}\,\]
3) Now we have to apply the criss cross method like shown below.
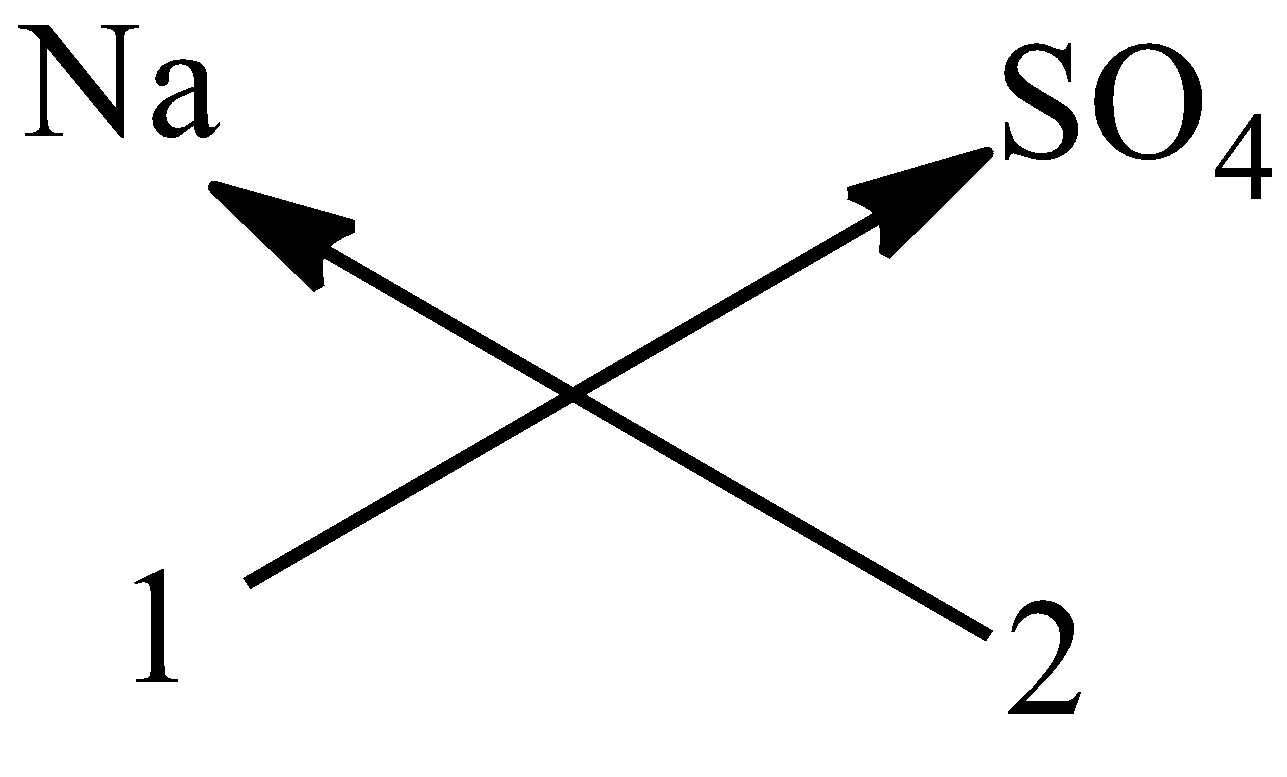
- Means the number on the bottom of the element is going to multiple with the opposite element to get the chemical formula of the compound.
- Therefore the chemical formula of the sodium sulphate as per criss cross method is $N{{a}_{2}}S{{O}_{4}}$ .
Note:
By using criss cross method we can write chemical formulas of all the inorganic chemicals. This method criss cross method is not applicable to organic compounds because of the absence of the cations and anions in organic compounds.
Complete answer:
- In the question it is asked to write the chemical formula of the sodium sulphate by using the criss cross method.
- Few steps we have to follow to write the chemical formula of the sodium sulphate and they are as follows.
1) We have to identify the cation and anion of the chemical and have to write the cation symbol first and later anion method like as follows.
- In the chemical compound we know that sodium is cation and sulphate is anion.
\[\underset{Cation}{\mathop{\text{Na }}}\,\text{ }\underset{Anion}{\mathop{\text{S}{{\text{O}}_{\text{4}}}}}\,\]
2) We have to write the charge of each ion at their bottom like this:
\[\underset{1\text{ }}{\mathop{\underset{Cation}{\mathop{\text{Na }}}\,\text{ }}}\,\text{ }\underset{\text{2}}{\mathop{\text{ }\underset{Anion}{\mathop{\text{S}{{\text{O}}_{\text{4}}}}}\,}}\,\]
3) Now we have to apply the criss cross method like shown below.

- Means the number on the bottom of the element is going to multiple with the opposite element to get the chemical formula of the compound.
- Therefore the chemical formula of the sodium sulphate as per criss cross method is $N{{a}_{2}}S{{O}_{4}}$ .
Note:
By using criss cross method we can write chemical formulas of all the inorganic chemicals. This method criss cross method is not applicable to organic compounds because of the absence of the cations and anions in organic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




