
Write down all possible functional isomers of $ {C_4}{H_8}O $ ?
Answer
512.4k+ views
Hint: In the question, it is asked to draw the possible functional isomers of $ {C_4}{H_8}O $ . The isomers are compounds which have the same chemical formula but they have different structural formulas. The phenomenon is termed as isomerism.
Complete answer:
We know that the compounds which are represented by the same molecular formula but arranged in different structures are called isomers and this phenomenon is called isomerism. In other words, we can say that the compounds which show isomerism phenomena are called isomers.
Isomers not only have different structures but they possess different physical and chemical properties as well. Isomerism can be classified into two categories i.e. structural isomerism and stereoisomerism.
Structural isomerism- The compounds which have differences in their structure come in the category of structural isomerism. The structural isomerism is further subdivided into chain, functional, positional, metamerism, tautomerism and ring- chain isomerism.
Stereoisomerism- The compounds which have differences in their spatial arrangement of atoms comes in the category of stereoisomerism. The stereoisomerism is further subdivided into two parts i.e. geometrical isomerism and optical isomerism.
Here, in the question we are asked to draw the functional isomerism. Let’s first understand functional isomerism.
Functional isomerism- The compounds which have the same chemical formula, but have different functional groups are called functional isomers and this phenomenon is called functional isomerism.
For the above chemical formula $ {C_4}{H_8}O $ there are a total of eight possible functional groups which include aldehyde, ketone, alcohol and ether.
The functional isomers possible from $ {C_4}{H_8}O $ are as follows:
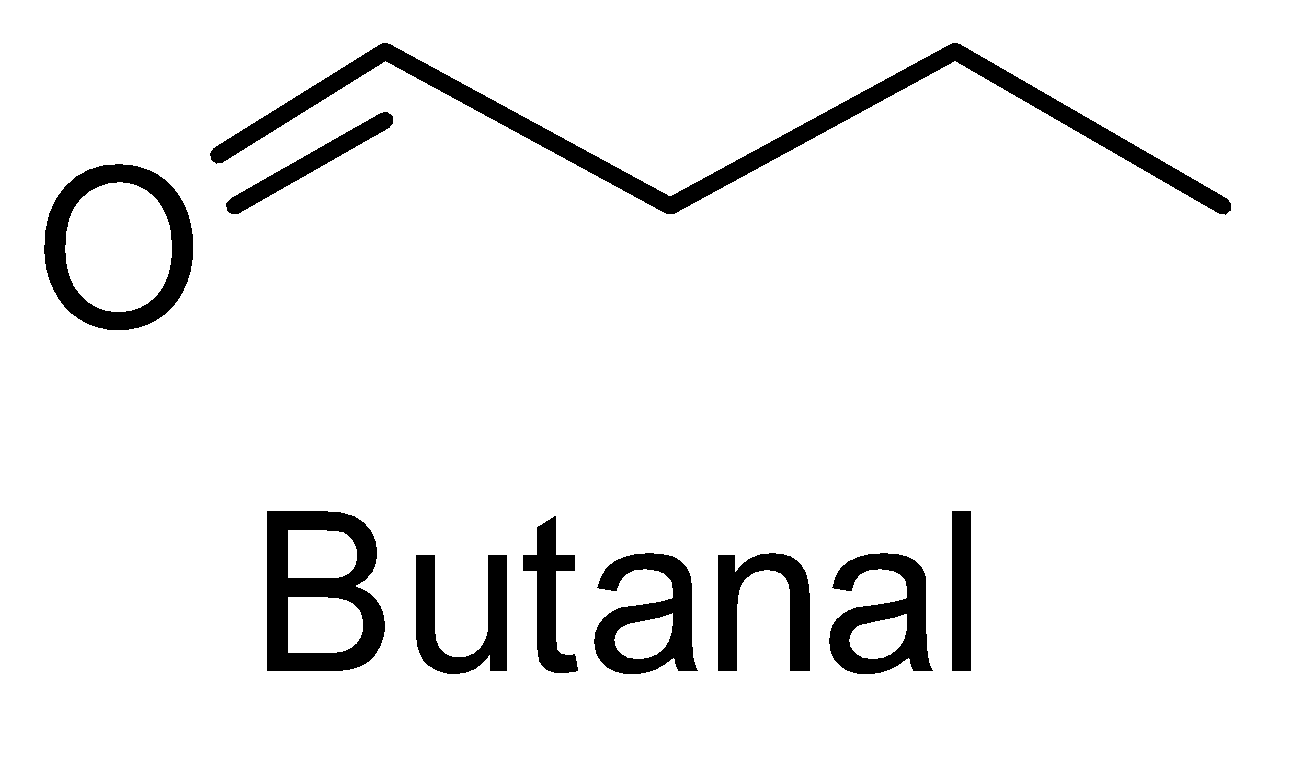
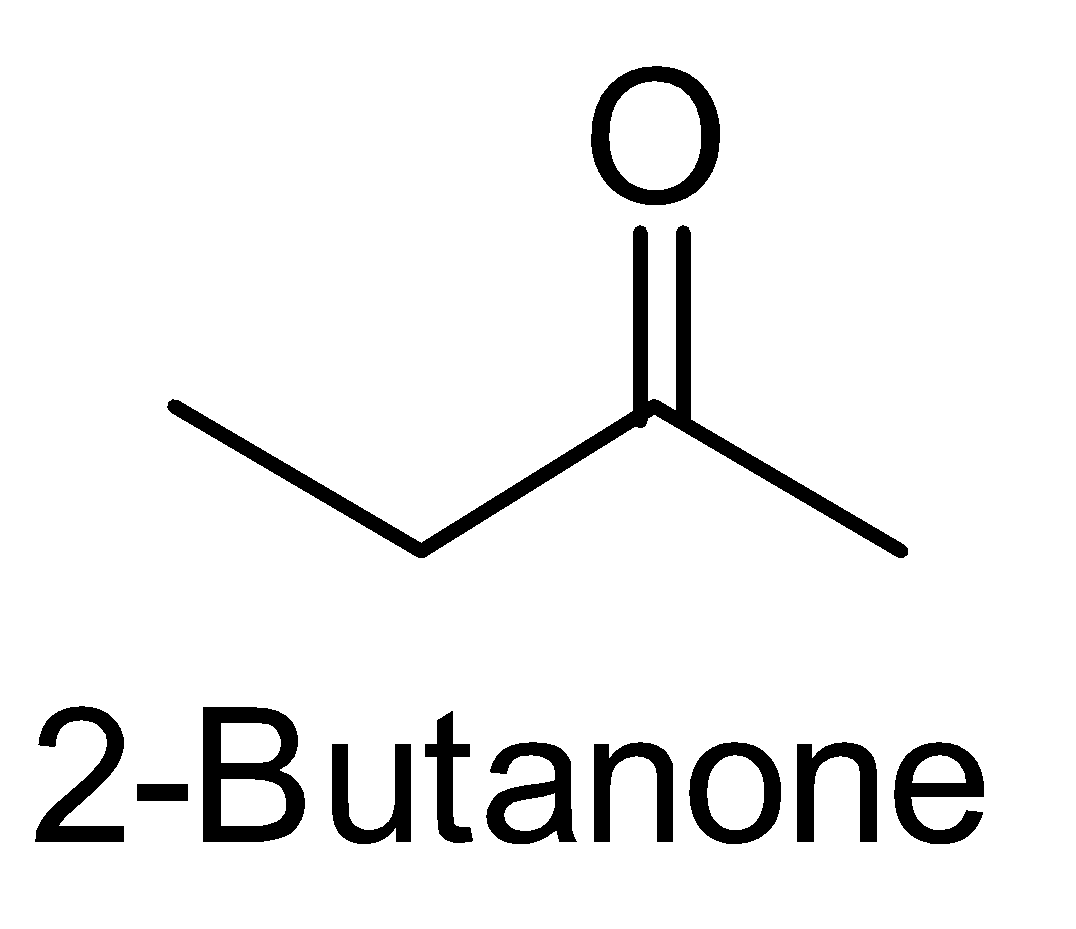
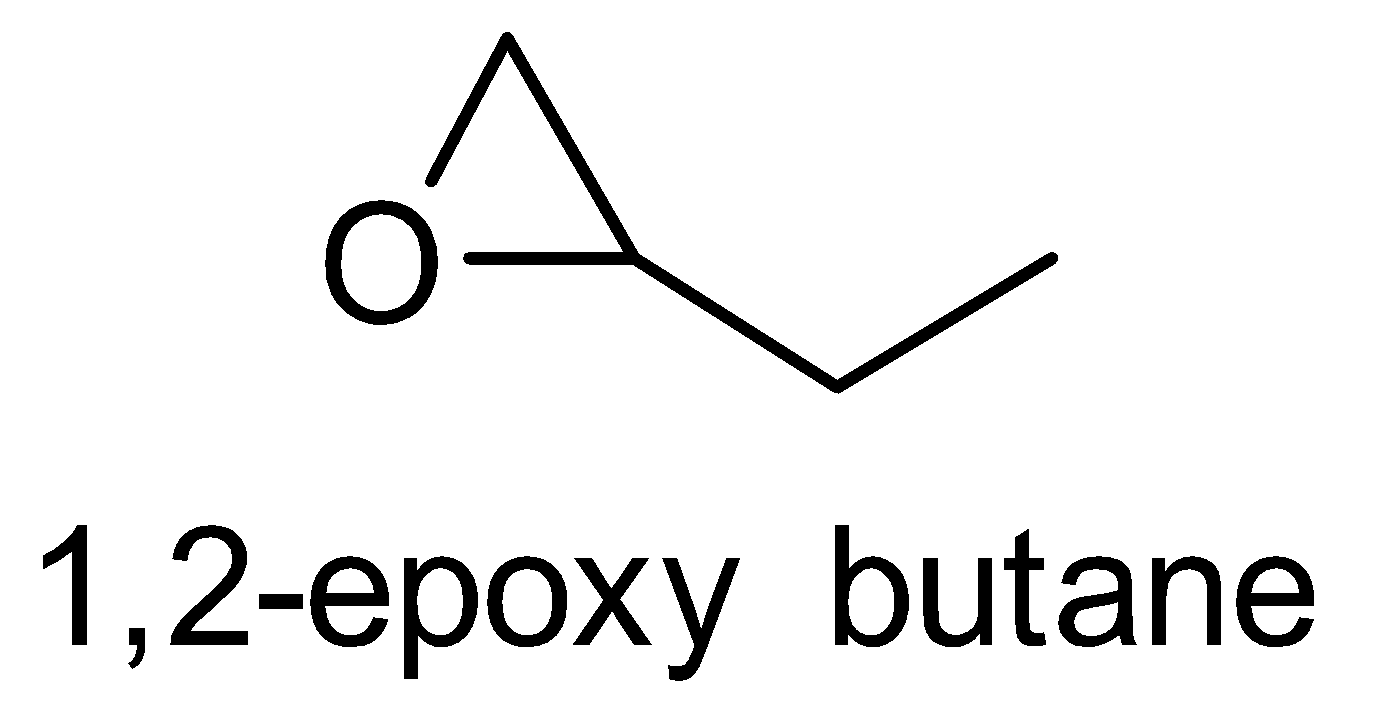
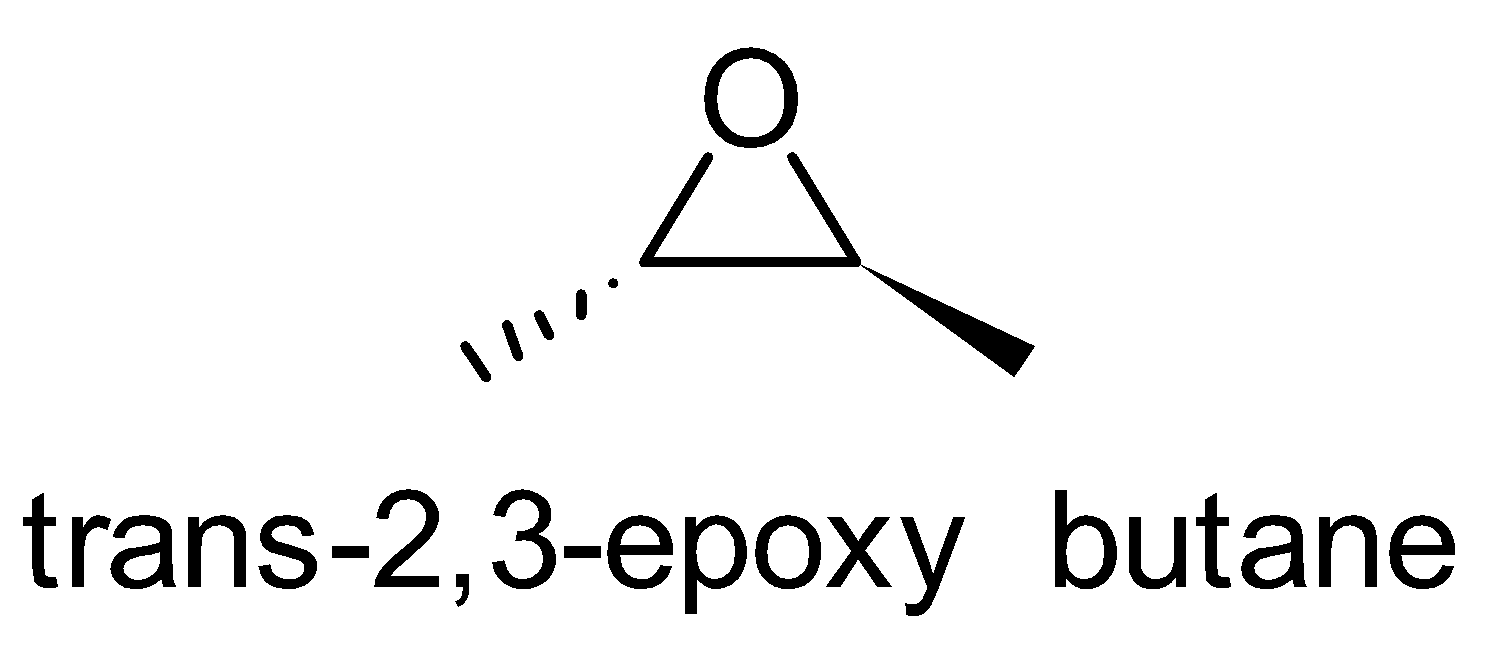
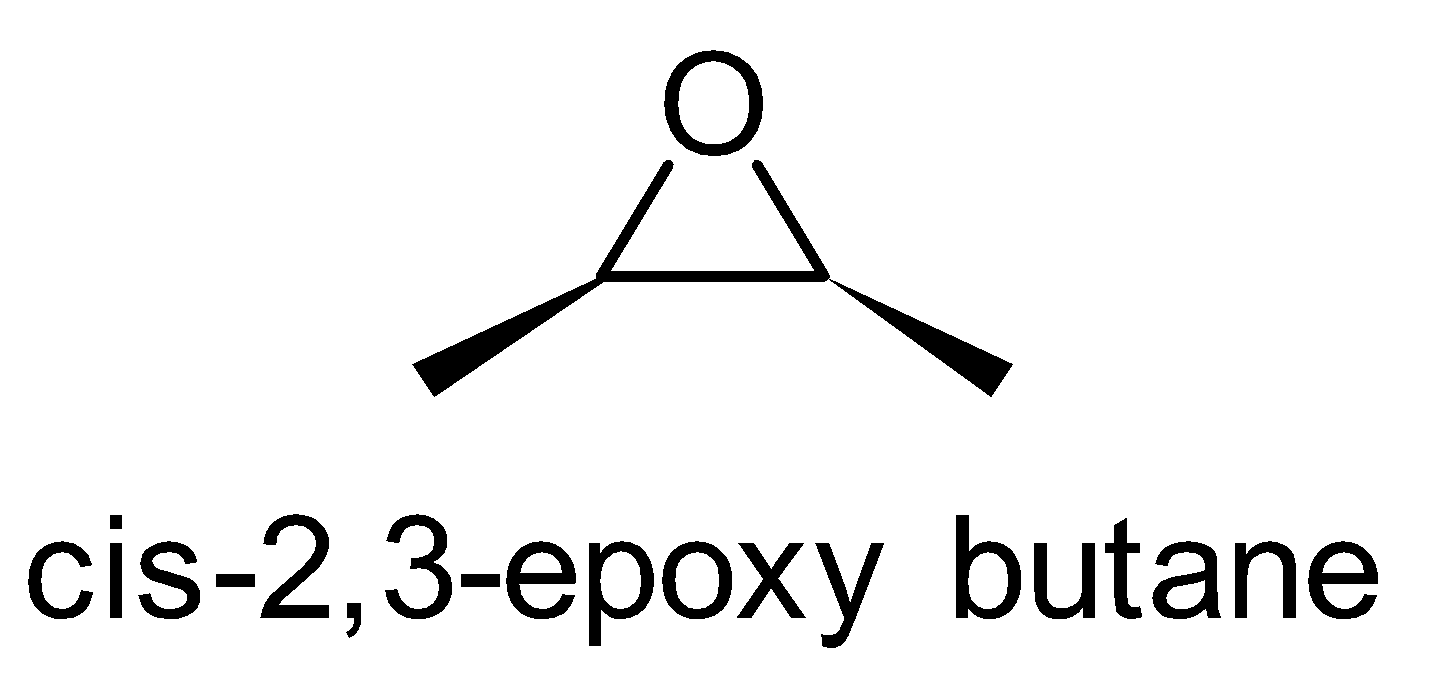
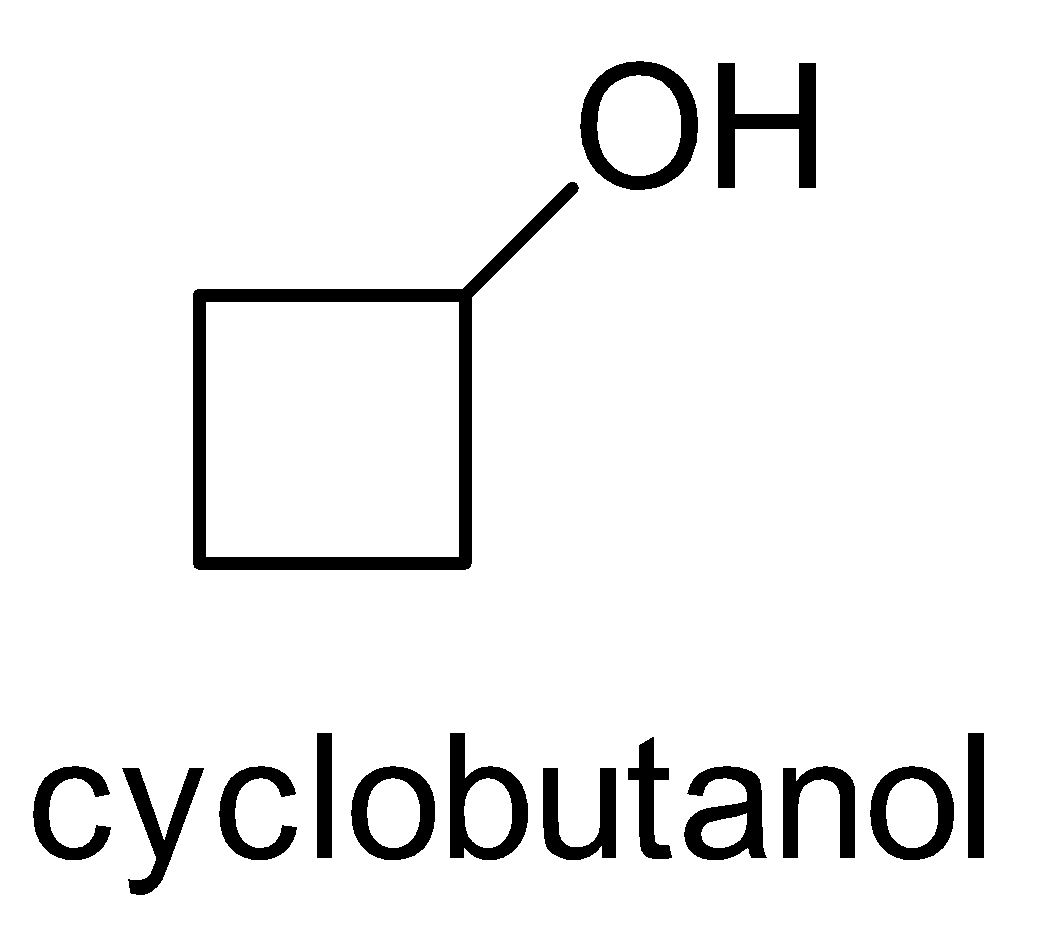
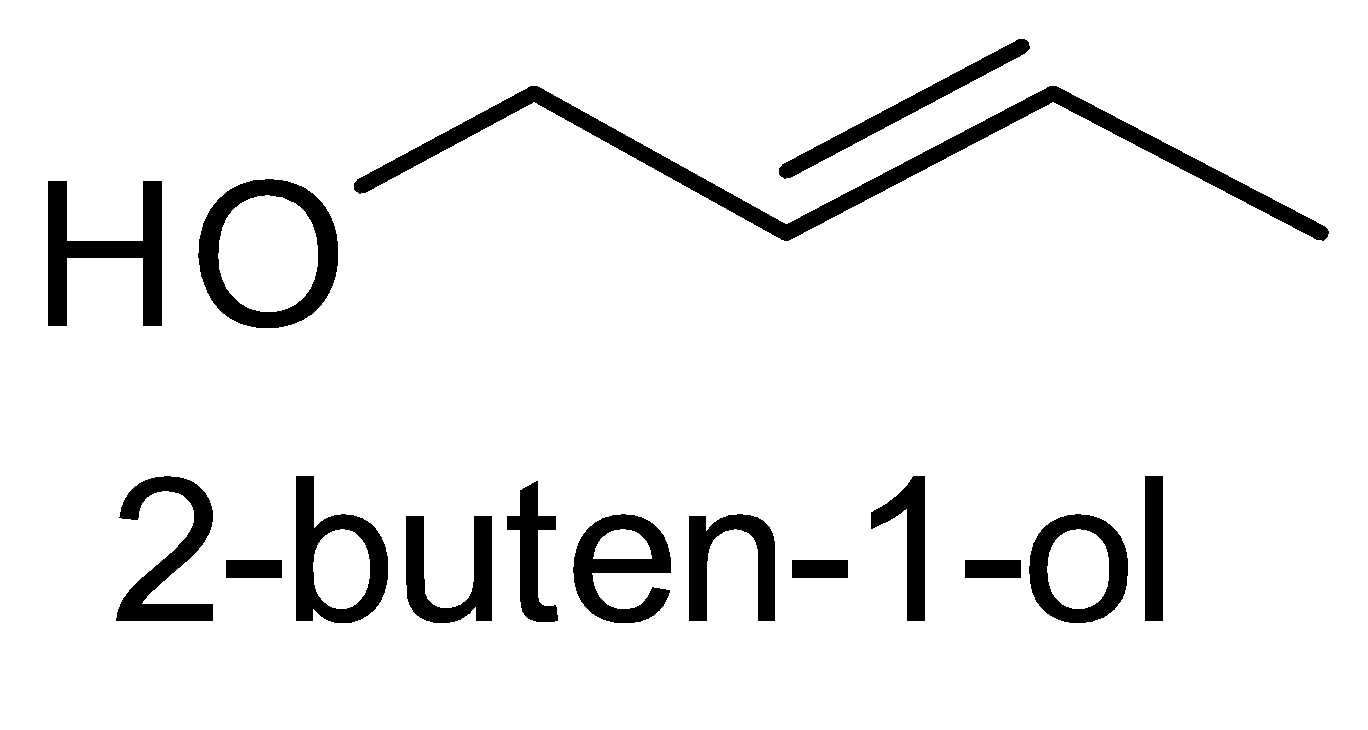
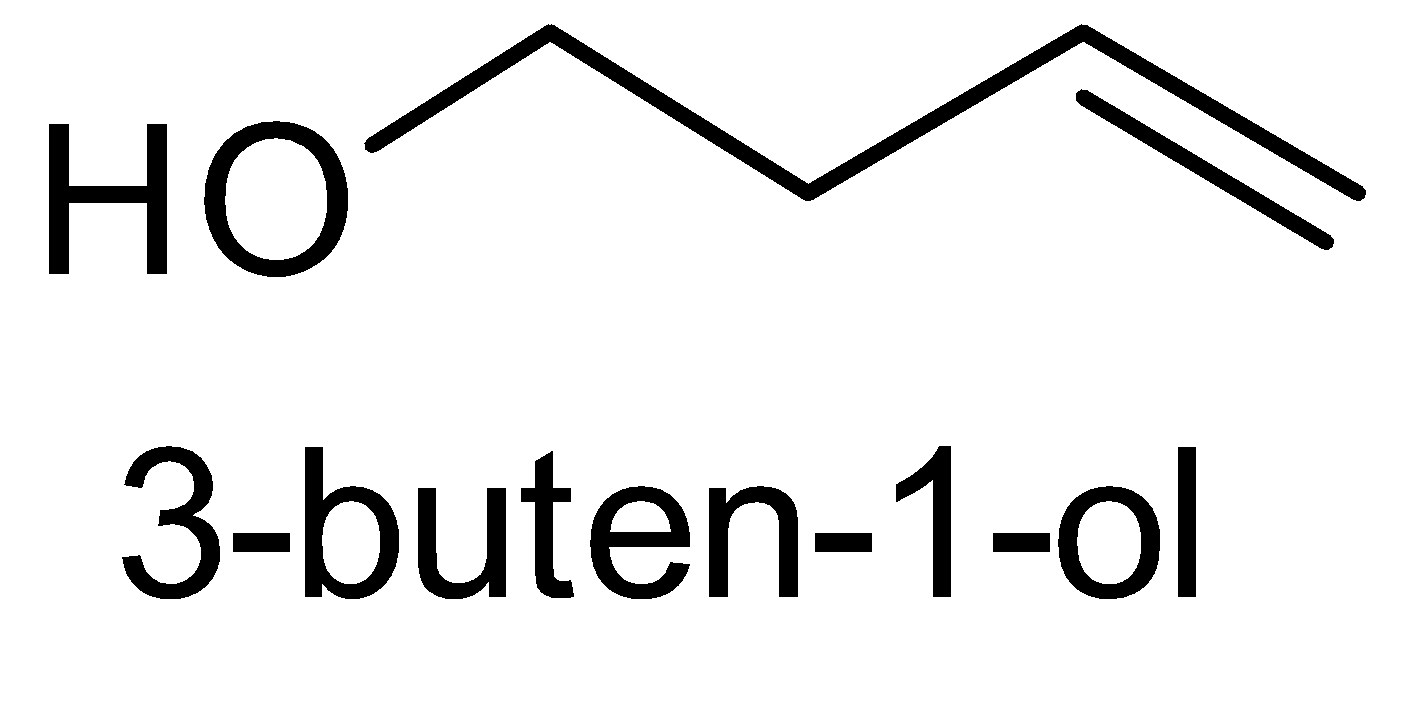
Hence there are eight possible functional isomers of $ {C_4}{H_8}O $
Note:
Functional isomers are the sub part of structural isomers. Functional isomerism occurs when there is difference in the arrangement of functional groups. The repeated structural formula of the same compound does not count as isomers.
Complete answer:
We know that the compounds which are represented by the same molecular formula but arranged in different structures are called isomers and this phenomenon is called isomerism. In other words, we can say that the compounds which show isomerism phenomena are called isomers.
Isomers not only have different structures but they possess different physical and chemical properties as well. Isomerism can be classified into two categories i.e. structural isomerism and stereoisomerism.
Structural isomerism- The compounds which have differences in their structure come in the category of structural isomerism. The structural isomerism is further subdivided into chain, functional, positional, metamerism, tautomerism and ring- chain isomerism.
Stereoisomerism- The compounds which have differences in their spatial arrangement of atoms comes in the category of stereoisomerism. The stereoisomerism is further subdivided into two parts i.e. geometrical isomerism and optical isomerism.
Here, in the question we are asked to draw the functional isomerism. Let’s first understand functional isomerism.
Functional isomerism- The compounds which have the same chemical formula, but have different functional groups are called functional isomers and this phenomenon is called functional isomerism.
For the above chemical formula $ {C_4}{H_8}O $ there are a total of eight possible functional groups which include aldehyde, ketone, alcohol and ether.
The functional isomers possible from $ {C_4}{H_8}O $ are as follows:








Hence there are eight possible functional isomers of $ {C_4}{H_8}O $
Note:
Functional isomers are the sub part of structural isomers. Functional isomerism occurs when there is difference in the arrangement of functional groups. The repeated structural formula of the same compound does not count as isomers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




